Collaborator Showcase: Cindy Cai
Collaborator Showcase: Cindy Cai
Cindy X. Cai, M.D., is the Jonathan and Marcia Javitt Rising Professor of Ophthalmology at Johns Hopkins University and a retina specialist seeing patients at the Wilmer Eye Institute’s locations in the Baltimore, Maryland area. Her primary focuses are in medical and surgical retina treatments, including: diabetic retinopathy, diabetic macular edema, and age-related macular degeneration.
Cindy graduated summa cum laude with a major in biology at Columbia University and received her M.D. from the Columbia University College of Physicians and Surgeons in New York. She led one of the 2023 SOS Challenge studies, which highlighted every step of an OHDSI network study, including design, implementations, execution, and dissemination.
A co-lead of the Eyecare and Vision Research Workgroup, Cindy is currently leading another OHDSI network study focused on Semaglutide and Nonarteritic Anterior Ischemic Optic Neuropathy. The 2024 Titan Award for Clinical Applications honoree, she discusses her career journey, her experience running her first community network study, opportunities in vision research using real-world data, and plenty more in the latest collaborator spotlight.
Global Symposium Welcomes 470+ Collaborators, Focuses on the Collaboration of Evidence Generation at Scale and Educating Community on OHDSI Tools, Best Practices
 The 10th annual OHDSI Global Symposium brought together more than 470 global collaborators for three days of sharing research, building new connections and pushing forward our mission of improving health by empowering a community to collaboratively generate the evidence that promotes better health decisions and better care.
The 10th annual OHDSI Global Symposium brought together more than 470 global collaborators for three days of sharing research, building new connections and pushing forward our mission of improving health by empowering a community to collaboratively generate the evidence that promotes better health decisions and better care.
The session included plenaries on Clinical Insights from LEGEND-T2DM and the Value Proposition for Participating in OHDSI Network Studies like LEGEND-T2DM, panels on Lessons Learned on the LEGEND-T2DM Journey and the new JACC-OHDSI Partnership, and a closing talk on Collaborating on Evidence at Scale.
The Collaborator Spotlight highlighted 136 posters/demos/lightning talks, all of which can be found here. Research from the 2024 Global Symposium Collaborator Showcase is also being shared daily on OHDSI’s LinkedIn, Twitter/X and Instagram feeds as part of the #OHDSISocialShowcase. Videos and slides from the event, including the Tuesday tutorials, will be posted to the event homepage when available.
 2024 Edition of Our Journey Annual Report Presented During Symposium, Highlights Community/Data Growth, 2024 Collaborations & More
2024 Edition of Our Journey Annual Report Presented During Symposium, Highlights Community/Data Growth, 2024 Collaborations & More
The latest edition of “Our Journey: Where the OHDSI Community Has Been, and Where We Are Going” was distributed at the Global Symposium, and the online version is available on the OHDSI website.
This publication, which is in its fourth edition, highlights all aspects of the OHDSI community, including its mission and history, the collaborators and workgroups that power the community, the collaborative activities and the various research pillars, including data standards, methods research, open-source development and clinical applications. There is also a list of more than 730+ published studies from the community over the last decade.
The 2024 edition highlights updated totals for global collaborators (4,294 across 83 countries) and patients with data mapped to OMOP (974 million). There is a new section on educational resources for the community, as well as a closing letter focused on where OHDSI can go in its second decade.
OHDSI Announces 2024 Titan Award Winners During Global Symposium
Collaborator Spotlight: Yong Chen
 Dr. Yong Chen, Professor of Biostatistics, founded and directs the Computing, Inference, and Learning Lab (PENNCIL) at the University of Pennsylvania. The mission of the PENNCIL lab is to develop computational methods and software to transform real-world data into insights, to disseminate the methods and knowledge to research communities, and to bridge the gap from data to actionable health care.
Dr. Yong Chen, Professor of Biostatistics, founded and directs the Computing, Inference, and Learning Lab (PENNCIL) at the University of Pennsylvania. The mission of the PENNCIL lab is to develop computational methods and software to transform real-world data into insights, to disseminate the methods and knowledge to research communities, and to bridge the gap from data to actionable health care.
Yong, who has been leading methodological work within the OHDSI community for several years, is an Elected Fellow in both the American Statistical Association (2020) and the the American College of Medical Informatics (ACMI) (2023), and he earned the 2021 OHDSI Titan Award for Methodological Research. His research areas include real-world data, clinical evidence generation, learning health systems and healthcare delivery.
In the latest edition of the Collaborator Spotlight, Yong discusses his career journey, recent advances in methods research, how his students use OHDSI in their research, and more.
First India Symposium Set For October 5 in Mumbai; Wide Array Of Community Leaders Scheduled To Lead Event
Collaborator Spotlight: Polina Talapova
 Polina Talapova is the lead of the Medical Team at SciForce, based in Ukraine. She is also a OMOP CDM Consultant for Tufts Medical Center. As a medical doctor with a PhD in pathology, Polina has made significant contributions to the OHDSI Vocabulary team. Since 2017, she has played a key role in developing mappings and machinery for an extensive list of medical terminologies, including ICD9CM, ICDs, LOINC, ATC, CPT4, HCPCS, NCCD, CVX, OMOP Extension, LPDs and more. Her most notable work centers on the improvement of Condition, Measurement, and Drug domains.
Polina Talapova is the lead of the Medical Team at SciForce, based in Ukraine. She is also a OMOP CDM Consultant for Tufts Medical Center. As a medical doctor with a PhD in pathology, Polina has made significant contributions to the OHDSI Vocabulary team. Since 2017, she has played a key role in developing mappings and machinery for an extensive list of medical terminologies, including ICD9CM, ICDs, LOINC, ATC, CPT4, HCPCS, NCCD, CVX, OMOP Extension, LPDs and more. Her most notable work centers on the improvement of Condition, Measurement, and Drug domains.
Polina is an active member of various working groups within OHDSI, including Vocabulary, Psychiatry, Vaccine, GIS, CDM, and THEMIS. Her extensive experience in ontology engineering, ETL and semantic mapping has led to the development of numerous validity checks and the authoring of substantial OHDSI Vocabulary documentation.
She discusses her career journey, OHDSI standardized vocabularies, her passion to build a Ukraine National Node and more in the latest collaborator spotlight.
Asia-Pacific Symposium Will Be Held Dec. 4-8 in Singapore; Registration Info Coming Soon
 We are excited to announce that the 2024 OHDSI APAC Symposium will be held in Singapore at the Marina Bay Sands and the National University of Singapore (NUS). This year’s event will be co-hosted with Singapore’s International Medical AGI Network Event (IMAGINE) and will feature a 1-day tutorial, a 2-day main conference and a 2-day datathon.
We are excited to announce that the 2024 OHDSI APAC Symposium will be held in Singapore at the Marina Bay Sands and the National University of Singapore (NUS). This year’s event will be co-hosted with Singapore’s International Medical AGI Network Event (IMAGINE) and will feature a 1-day tutorial, a 2-day main conference and a 2-day datathon.
More information is available at the event homepage, including a tentative agenda and key dates leading up to the APAC Symposium. When registration opens, it will be linked on the event page and announced on OHDSI channels and the community call.
Tutorials, Conference Agenda, Workgroup Activities Announced For 2024 OHDSI Global Symposium
 Registration is open for the 2024 OHDSI Global Symposium, which will be held October 22-24 at the Hyatt Regency Hotel in New Brunswick, N.J., USA. The event will include a day of tutorials, a day of plenaries and the collaborator showcase, and a day of workgroup activities. Check out the event homepage for more information.
Registration is open for the 2024 OHDSI Global Symposium, which will be held October 22-24 at the Hyatt Regency Hotel in New Brunswick, N.J., USA. The event will include a day of tutorials, a day of plenaries and the collaborator showcase, and a day of workgroup activities. Check out the event homepage for more information.
The first day of the 2024 OHDSI Global Symposium will feature five tutorial options for the community. During the morning (8 am – 12 pm), there will be one tutorial that is designed for newcomers but can be useful to anybody in the community: An Introduction to the Journey from Data to Evidence Using OHDSI. During the afternoon (1 pm – 5 pm), there will be four concurrent tutorials with specialized focuses: (1) An Introduction to the Journey from Data to Evidence using OHDSI; (2) Developing and Evaluating Your Extract, Transform, Load (ETL) Process to the OMOP Common Data Model; (3) So, You Think You Want To Run an OHDSI Network Study?; and (4) Using the OHDSI Standardized Vocabularies for Research. You can select your tutorials during the registration process.
The full agenda for the main conference (Wednesday) and the schedule for workgroup activities (Thursday) is also posted on the Global Symposium homepage. You can register now for the event and individual tutorials; each will be capped at a designated total, based on the size of the available room, so please don’t wait until the last minute.
Collaborator Spotlight: Jason Hsu
Europe Symposium Highlights How Collaborators Are Scaling up Reliable Evidence; All Videos, Slides, Posters & Tutorials Are Now Available
 The fifth European OHDSI Symposium, titled “Scaling up Reliable Evidence Across Europe,” was held June 1-3 and brought together data partners, regulators, and researchers to collaborate and share results and ideas about the use of the OMOP-CDM in Europe. During the main conference, there were sessions focused on the Selection of European Initiatives Using the OMOP CDM (Session 1, 42:45) and Large Scale Evidence Generation in EHDEN and DARWIN EU® (Session 3, 6:55), as well as Rapid-Fire Presentations and posters/software demonstrations from the collaborator showcase.
The fifth European OHDSI Symposium, titled “Scaling up Reliable Evidence Across Europe,” was held June 1-3 and brought together data partners, regulators, and researchers to collaborate and share results and ideas about the use of the OMOP-CDM in Europe. During the main conference, there were sessions focused on the Selection of European Initiatives Using the OMOP CDM (Session 1, 42:45) and Large Scale Evidence Generation in EHDEN and DARWIN EU® (Session 3, 6:55), as well as Rapid-Fire Presentations and posters/software demonstrations from the collaborator showcase.
There were also multiple tutorials and workshops held throughout the weekend, including a two-part vocabulary workshop entitled What you need to know about OHDSI Vocabularies to do phenotyping? (part 1 | part 2). There were workshops focused on open-source development, phenotyping and HADES.
All materials from the 2024 Europe Symposium have now been posted, and you can access them all through the main event homepage.
Collaborator Spotlight: Sarah Seager
Sarah Seager is the Senior Director, Analytics & AI at IQVIA. She is an experienced technical senior leader, who develops and leads analytical teams in the world of data science and advanced analytics.
Having built a career mainly within the health sector over a period of 20 plus years, Sarah started her career in the world of data as a data input clerk, working her way up into the realms of senior analytics and then into data management and data science. She has been a collaborator within the OHDSI community since 2018 and has been an active contributor in symposiums around the world.
In the latest edition of the Collaborator Spotlight, Sarah shares her thoughts on the current path of data management and analytics, why OMOP is an ideal common data model, recent advances around the European community, and how data is similar to another one of Sarah’s passions, art.
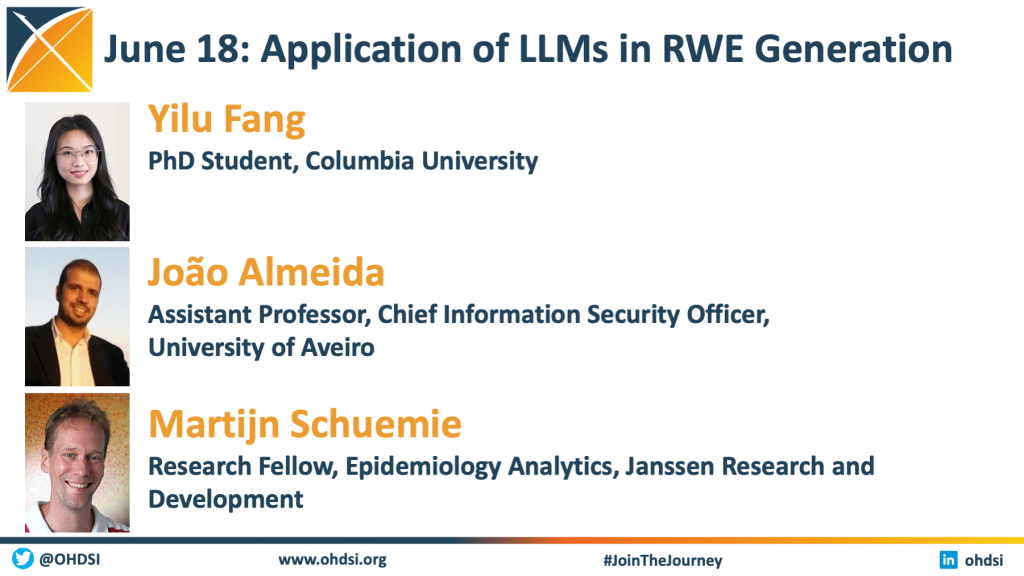 Large Language Models Can Enhance OHDSI Evidence Generation Mission
Large Language Models Can Enhance OHDSI Evidence Generation Mission
Large language models can analyze large datasets, extract insights, and generate evidence-based reports, aiding in real-world decision-making by providing accurate, comprehensive information efficiently. The OHDSI Generative AI and Foundational Models workgroup focuses on advancing healthcare research and improve patient outcomes through the innovative application of generative AI and foundational models. If you are interested in learning more about this new workgroup or would like to join in its upcoming meetings, please fill out this form.
Three members of the OHDSI global community joined the June 18 community call to present recent research in the area of large language models. You can find the video and slides for each presentation here.
OHDSI Evidence Network Update: New Network Study, Protocol, Information on How To Join This Growing Research Asset
 The OHDSI community is passionate about generating evidence to support better health decisions and better care. To carry out our mission, we need an active and willing global network of data partners, and we need the ability to quickly identify those that might be the right fit for a specific clinical research question. Last year we piloted this effort through the Save our Sisyphus challenge and are now ready to move forward based on our learnings. If you would like to learn more please see this video from the 2023 Global Symposium, or this update from the June 11 community call.
The OHDSI community is passionate about generating evidence to support better health decisions and better care. To carry out our mission, we need an active and willing global network of data partners, and we need the ability to quickly identify those that might be the right fit for a specific clinical research question. Last year we piloted this effort through the Save our Sisyphus challenge and are now ready to move forward based on our learnings. If you would like to learn more please see this video from the 2023 Global Symposium, or this update from the June 11 community call.
The OHDSI Evidence Network workgroup is excited to initiate a network study that will describe the OHDSI Network in a publication, and will also create an open public resource designed to facilitate evidence generation faster and better than ever by building on methodologies developed by thought leaders around the world.
You can access the protocol here. Come join us on this exciting journey!
Collaborator Spotlight: Ajit Londhe 
Ajit Londhe is a Senior Director in the RWE Analytics team at Boehringer Ingelheim. He received his Bachelor of Science in Computer Science from Penn State University and his Master’s in Public Health from Emory University.
Ajit contributes to various OHDSI software projects, such as Atlas, HADES, and Broadsea. His OHDSI studies typically involve population level estimation utilizing secondary data sources such as administrative claims and EHRs. Ajit won the 2022 OHDSI Titan Award for Community Collaboration.
In the latest edition of the Collaborator Spotlight, Ajit discusses his career journey, the value of open-source development to OHDSI’s mission, some of the exciting new tools being built in the community, and plenty more.
New “10-Minute Tutorials” Highlight Potential Impact from Four Community-Developed and Maintain Open-Source Tools To Aid Community Research
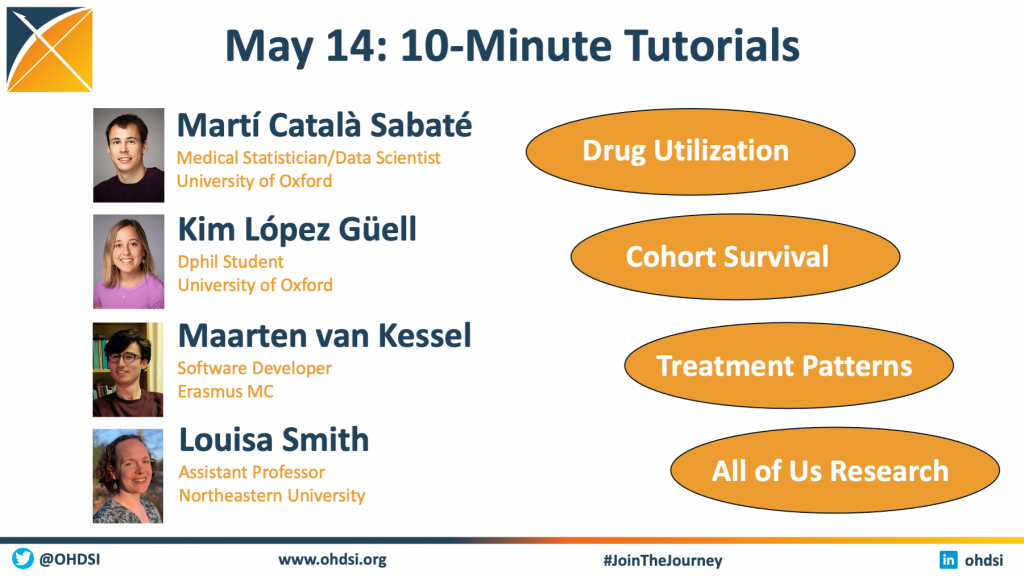 The May 14 community call featured a series of 10-minute tutorials, as four open-source development leaders provide introductions and brief live demonstrations on tools that can aid our community in observational healthcare research:
The May 14 community call featured a series of 10-minute tutorials, as four open-source development leaders provide introductions and brief live demonstrations on tools that can aid our community in observational healthcare research:
• Martí Català Sabaté, Medical Statistician/Data Scientist, University of Oxford (Drug Utilization)
• Kim López Güell, Dphil Student, University of Oxford (Cohort Survival)
• Maarten van Kessel, Software Developer, Erasmus MC (Treatment Pattens)
• Louisa Smith, Assistant Professor, Northeastern University (All of Us Research)
Each tutorial is available on the OHDSI Community Calls page, as well as our open-source tutorials page, which includes a variety of tutorials that have been shared over the last few years.
Kheiron Cohort Onboards New Software Developers; Applications Being Accepted Through June 1
Open-Source Community workgroup co-lead Paul Nagy announced that applications for the 2024-25 Kheiron Cohort are now open and will be accepted through June 1. The program is designed to onboard new contributors into OHDSI to empower them to become active contributors and maintainers. The goals are to: 1) provide career development — including training opportunities within the cohort from OHDSI technical leaders, as well as mentoring from OHDSI leadership — 2) create a global perspective of the ecosystem; 3) build cross-connections between projects; and 4) develop future leaders for OHDSI.
Katy Sadowski was a member of the original Kheiron Cohort in 2022-23, and she quickly became a leader in the OHDSI open-source community. A recipient of the 2023 Titan Award for Open-Source Development. Sadowski is now a HADES maintainer and Kheiron faculty member. She discussed the program during a recent community call.
Registration Is OPEN For The 2024 Global Symposium, Held Oct. 22-24 at the Hyatt Regency Hotel in New Brunswick, N.J., USA
 Registration is open for the 10th annual OHDSI Global Symposium, which will be held in person October 22-24 at the Hyatt Regency Hotel in New Brunswick, N.J., USA. We are planning another exciting three-day event. On Tuesday, October 22, we will have space for open collaboration for the community as well as multiple tutorials, including an Introduction to OHDSI session in the morning and four advanced tutorials in the afternoon. On Wednesday, October 23, we will host plenaries and our annual collaborator showcase. On Thursday, October 24, the collaborator showcase will continue along with workgroup activities throughout the day.
Registration is open for the 10th annual OHDSI Global Symposium, which will be held in person October 22-24 at the Hyatt Regency Hotel in New Brunswick, N.J., USA. We are planning another exciting three-day event. On Tuesday, October 22, we will have space for open collaboration for the community as well as multiple tutorials, including an Introduction to OHDSI session in the morning and four advanced tutorials in the afternoon. On Wednesday, October 23, we will host plenaries and our annual collaborator showcase. On Thursday, October 24, the collaborator showcase will continue along with workgroup activities throughout the day.
 Collaborator Spotlight: Montse Camprubi
Collaborator Spotlight: Montse Camprubi
Montse Camprubi works at Synapse Research Management Partners. She has wide experience in managing and coordinating complex research projects as well as acting as community manager, including the development of internal communication mechanisms to promote communication dynamics. Montse is highly skilled in EU funded projects with deep knowledge on financing and control resource procedures.
Currently the EHDEN community manager, she is leading the central coordination efforts between EHDEN Data Partners and certified SMES and EHDEN experts.
In the latest edition of the collaborator spotlight, Montse discusses her background and career journey, recent highlights and future plans in EHDEN, the upcoming OHDSI Europe Symposium, and plenty more.
DevCon 2024 (April 26) Brings Together Open-Source Community, Envisions Potential, Possibilities of OHDSI Software
Maternal Health Data Science Fellowship Opportunity Will Focus On Career Development, Practice & Networking For Early-Stage Researchers; Application Deadline is May 15
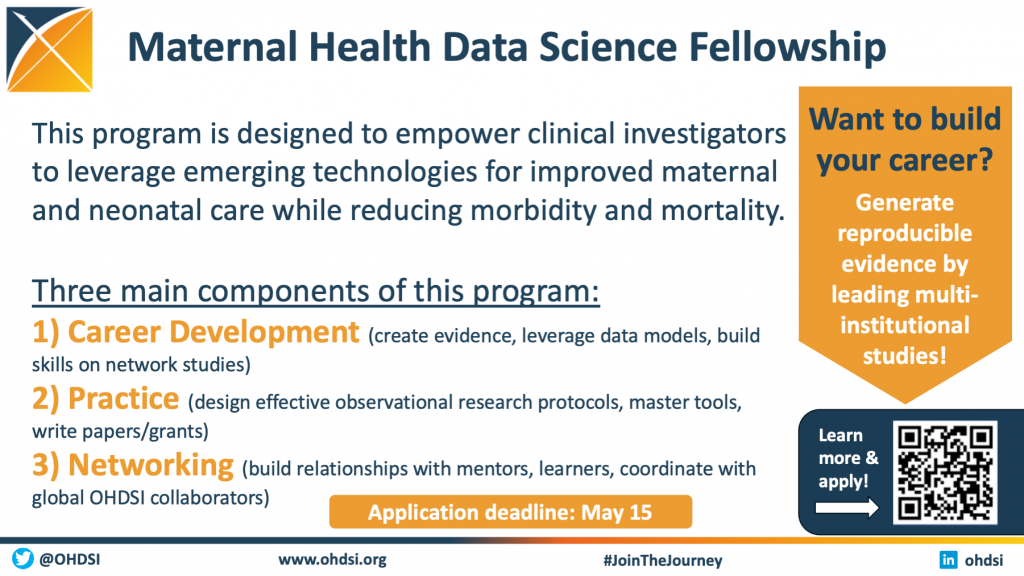 The Perinatal and Reproductive Health workgroup is collaborating with other OHDSI leaders to lead the Maternal Health Data Science Fellowship, a nine-month program designed to empower clinical investigators to leverage emerging technologies for improved maternal and neonatal care while reducing morbidity and mortality.
The Perinatal and Reproductive Health workgroup is collaborating with other OHDSI leaders to lead the Maternal Health Data Science Fellowship, a nine-month program designed to empower clinical investigators to leverage emerging technologies for improved maternal and neonatal care while reducing morbidity and mortality.
The program, which focuses on the key components of career development, practice and networking, will train clinical investigators in observational research methods to enable them to conduct reproducible research and generate real-world evidence. The leadership team aims to guide students in the production of multi-institutional network studies and help them win future grants.
Candidates will be able to leverage the NIH Maternal Health OHDSI Data Partner Network, comprised of seven US-based Academic Medical Centers. Network studies led by candidates will be based on this network and be open to any data partners in the OHDSI Evidence Network. This program is designed for early-stage researchers in academic medicine who are focusing their career on creating evidence from real world data in Maternal Health. Candidates will be given preference if their organization participates in the OHDSI data network and/or the NIH Inspire initiative. More information is available, and all applications are due by May 15.
Collaborator Spotlight: Melanie Philofsky
Yong Chen Leads Next CBER BEST Seminar Series (Apr. 17, 11 am ET); Discussion Will Focus On Vaccine Effectiveness, Causal Inference
 The CBER BEST Initiative Seminar Series returns Wednesday, April 17 (11 am – 12 pm ET) as 2021 Titan Award honoree Yong Chen presents his research on “Real-World Effectiveness of BNT162b2 Against Infection and Severe Diseases in Children and Adolescents: causal inference under misclassification in treatment status.” This series is open to anybody: Calendar invite to CBER BEST Seminar.
The CBER BEST Initiative Seminar Series returns Wednesday, April 17 (11 am – 12 pm ET) as 2021 Titan Award honoree Yong Chen presents his research on “Real-World Effectiveness of BNT162b2 Against Infection and Severe Diseases in Children and Adolescents: causal inference under misclassification in treatment status.” This series is open to anybody: Calendar invite to CBER BEST Seminar.
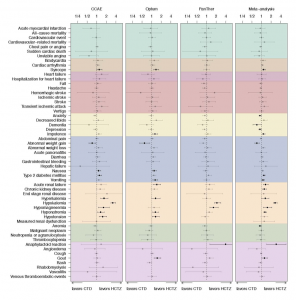
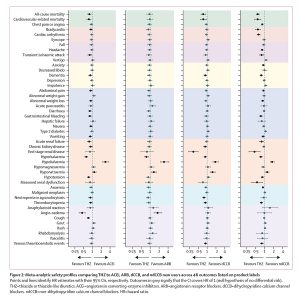
Overview: The current understanding of long-term effectiveness of the BNT162b2 vaccine across diverse U.S. pediatric populations is limited. We assessed the effectiveness of BNT162b2 against various strains of the SARS-CoV-2 virus using data from a national collaboration of pediatric health systems (PEDSnet). We emulated three target trials to assess the real-world effectiveness of BNT162b during the Delta and Omicron variant periods. In the U.S., immunization records are often captured and stored across multiple disconnected sources, resulting in incomplete vaccination records in patients’ electronic health records (EHR). We implemented a novel trial emulation pipeline accounting for possible misclassification bias in vaccine documentation in EHRs. The effectiveness of the BNT162b2 vaccine was estimated from the Poisson regression model with confounders balanced via propensity score stratification. This study suggests BNT162b2 was effective among children and adolescents in Delta and Omicron periods for a range of COVID-19-related outcomes and is associated with a lower risk for cardiac complications.
April Olympians Collab-A-Thon Will Focus On CDM and THEMIS Conventions, Documentation; Community Calls Will Include Presentations Around The ETL Process
As the OHDSI community grows and use of the OMOP CDM expands — OMOP currently has 534 global data sources mapped with 956 million unique patient records available for research — there is a need for clear, concise and easily accessible documentation on the CDM and THEMIS conventions required to properly standardize data
This month, Clair Blacketer and Melanie Philofsky are leading a collaborative activity called “April Olympians,” which will serve to (1) identify all currently ratified CDM and THEMIS conventions for every CDM table and field; (2) write clear documentation for each THEMIS convention; (3) establish a repository for THEMIS conventions; (4) update the CDM documentation to link to relevant THEMIS repository entries; and (5) create CDM documentation related to expansion module efforts around the community. There is a public Github repo to manage all activities; if you are interested in joining this effort, please fill out this form.
As this collab-a-thon progresses through April, the weekly community calls will focus on different aspects of the ETL process. From technical considerations and vocabularies to open-source tools and the varying processes to ratify new THEMIS conventions or create site-specific add-ons to the CDM, each community call will feature an information presentation following an April Olympians call update.
Winter 2024 OHDSI Standardized Vocabularies Release Announced
OHDSI standardized vocabularies allow organization & standardization of medical terms to be used across various clinical domains of the OMOP CDM for observational research. Beginning in 2023, the OHDSI vocabulary team makes two major releases annually, including domain changes, newly added concepts, standard concept changes, changes of concept mapping, and more. The most recent release was shared 29Feb2024, and it included updates in 12 groupings, which you can read about here.
Alexander Davydov, Oleg Zhuk and Anna Ostropolets each joined the first community call in March to provide a presentation on the update process, a closer look at some of the specific vocabulary updates, and a look at how the community contribution process provided 11 specific enhancements, with more to come in the August 2024 release.
You can check out the presentation slidedeck for more details on the release. Thank you to all community members on the vocabulary team for all the work you put into this release!
Collaborator Showcase: Ross Williams
 Ross Williams is a scientific researcher working in the group of Dr. Peter Rijnbeek at Erasmus MC, where he is part of the Health Data Science group. His main focus is creating tools and analysis methods to develop personalised medical risk prediction. His specific areas of interest are on the external validation of prediction models, net benefit assessment and techniques for temporal health data analysis. He co-leads both the Patient Level Prediction workgroup and the Early-Stage Researcher workgroup.
Ross Williams is a scientific researcher working in the group of Dr. Peter Rijnbeek at Erasmus MC, where he is part of the Health Data Science group. His main focus is creating tools and analysis methods to develop personalised medical risk prediction. His specific areas of interest are on the external validation of prediction models, net benefit assessment and techniques for temporal health data analysis. He co-leads both the Patient Level Prediction workgroup and the Early-Stage Researcher workgroup.
A 2021 Titan Award honoree, Ross obtained his PhD at Erasmus University Medical Center (2023) and his MSc (2017) in Data Science from King’s College London. He previously obtained his BSc in Physics and Philosophy from the same institution. Before starting work at Erasmus MC he spent time working on a Marie Curie scholarship on the TRANSACT project under the EU FP7 initiative.
Ross discusses his career journey, how observational data impacts prediction models, the opportunities for junior researchers in OHDSI, and plenty more in the latest edition of the Collaborator Spotlight.
Registration Opens For 2024 Europe Symposium (June 1-3, Rotterdam, Netherlands); Collaborator Showcase Deadline Set For March 15
 Registration has opened for the fifth European OHDSI Symposium, which will be held June 1-3 in Rotterdam, Netherlands. The symposium, which is entitled ‘Scaling up reliable evidence across Europe’, will feature two days of workshops and tutorials, and then the main conference will be held June 3 on the Steam Ship Rotterdam.
Registration has opened for the fifth European OHDSI Symposium, which will be held June 1-3 in Rotterdam, Netherlands. The symposium, which is entitled ‘Scaling up reliable evidence across Europe’, will feature two days of workshops and tutorials, and then the main conference will be held June 3 on the Steam Ship Rotterdam.
The symposium is a platform to share results and ideas about the use of the OMOP-CDM, tool development, and future research. The day will include a collaborator showcase including posters and podium presentations to highlight OHDSI’s research achievements, and interactive demonstrations of OHDSI’s open-source software tools. The deadline to submit brief reports for the collaborator showcase is March 15.
During the symposium, there will be updates on the exciting work being done all over Europe, including in National Nodes, large European Projects, and other initiatives. There will also be plenty of time for networking. More information and a registration link is available on the event homepage.
Advancing Phenotype Science and Driving Collaborative Research Around Four Phenotypes Serve As Foundation For Phenotype Phebruary 2024
Collaborator Spotlight: Kerry Goetz

Kerry Goetz is the Associate Director for the National Eye Institute’s Office of Data Science and Health Informatics at the US National Institutes of Health. In this capacity she is responsible for advancing data management and sharing strategies to make NEI data FAIR (Fully AI-Ready & Findable, Accessible, Interoperable, and Reusable). For over a decade, Kerry has been leading the eyeGENE Program, a controlled access resource with imaging, data, samples, and a participant registry for rare eye conditions. Kerry has also been entrenched in standards development for over 15 years.
Kerry co-leads the Eye Care and Vision Research Observational Health Data Sciences and Informatics Working Group, is a member of the American Academy of Ophthalmology Standards Working Group, and also works to aligning imaging standards and health data to enable groundbreaking research. She is also a PhD Candidate at George Mason University, studying Health Services Research with a Knowledge Discovery and Health Informatics Concentration.
She discusses her career journey, evidence gaps around vision research, how OHDSI impacts her PhD journey, and more in the latest collaborator spotlight.
 First UK OHDSI Studyathon Focuses on Use of Fluoroquinolones Across Geographies/Time, Epidemiology and Characterization of Rectal Prolapse and Rectopexy
First UK OHDSI Studyathon Focuses on Use of Fluoroquinolones Across Geographies/Time, Epidemiology and Characterization of Rectal Prolapse and Rectopexy
The Jan. 23 community call looked at the recent UK Study-A-Thon, held last November at Saint Hilda’s College. The event focused on the use of fluoroquinolones across geographies and over time, as well as on the epidemiology and characterization of rectal prolapse and rectopexy. At the end of an intense week, the team generated three draft manuscripts almost ready for submission, and at least four conference abstracts were in the making.
Daniel Prieto-Alhambra, Katherine Donegan, Annika Jodicke, and Jennifer Lane each provided insight during the presentation, which opened with a look at the Medicines and Healthcare products Regulatory Agency and the use of real-world evidence. The full video presentation is available here, and the slidedeck is available here.
Collaborator Spotlight: Chungsoo Kim
 Chungsoo Kim is a PhD candidate in the Department of Biomedical Informatics at Ajou University College of Medicine. He earned his Doctor of Pharmacy degree from the College of Pharmacy of the same university in 2019. His research interests include reliable real-world evidence for medication and prediction of individual drug effects/adverse events based on the OMOP common data models. He is also interested in data/analytics infrastructure for conducting data-driven research.
Chungsoo Kim is a PhD candidate in the Department of Biomedical Informatics at Ajou University College of Medicine. He earned his Doctor of Pharmacy degree from the College of Pharmacy of the same university in 2019. His research interests include reliable real-world evidence for medication and prediction of individual drug effects/adverse events based on the OMOP common data models. He is also interested in data/analytics infrastructure for conducting data-driven research.
Since joining OHDSI in 2019, he has participated in and led several research projects at OHDSI. He currently participates in OHDSI working groups, including PatientLevelPrediction and the APAC group. He also served as a tutorial instructor for the 2019 OHDSI Korea International Symposium.
Chungsoo discusses his research focuses, his involvement in the OHDSI community, the growth of OHDSI around the Asia-Pacific region, and plenty more in the latest Collaborator Spotlight.
How Far We’ve Come: OHDSI Reflects On Its First Decade
 On Dec. 16, 2013, George Hripcsak led the official formation of the OHDSI community. Within a month, the first face-to-face meeting was held within the Department of Biomedical Informatics at Columbia University. How did we get from there to a global community of more than 3,800 collaborators? The Dec. 12 community call reflected on 10 years of OHDSI, with a video presentation led by Patrick Ryan.
On Dec. 16, 2013, George Hripcsak led the official formation of the OHDSI community. Within a month, the first face-to-face meeting was held within the Department of Biomedical Informatics at Columbia University. How did we get from there to a global community of more than 3,800 collaborators? The Dec. 12 community call reflected on 10 years of OHDSI, with a video presentation led by Patrick Ryan.
The presentation highlights several of the firsts in the community, including its first publication (which now has more than 1,000 citations), first symposia in the United States, Europe and the Asia-Pacific region, first open-source tools, and plenty more. It also reflects on some of the clinical impacts made by the OHDSI community.
The video presentation is available here, while the slidedeck (which includes the 2023 Year In Review slides) can be found here.
Collaborator Spotlight: Alison Callahan
 Alison Callahan is an Instructor in the Center for Biomedical Informatics and Clinical Data Scientist in the Stanford Health Care Data Science Team. Her current research uses informatics to extract perinatal health data from electronic health records, and to study medication usage and effectiveness in pregnancy. She is also the co-leader of the OHDSI Perinatal & Reproductive Health (PRHeG) working group. Her work in the SHC Data Science team focuses on developing and implementing methods to assess and identify high value applications of machine learning in healthcare settings.
Alison Callahan is an Instructor in the Center for Biomedical Informatics and Clinical Data Scientist in the Stanford Health Care Data Science Team. Her current research uses informatics to extract perinatal health data from electronic health records, and to study medication usage and effectiveness in pregnancy. She is also the co-leader of the OHDSI Perinatal & Reproductive Health (PRHeG) working group. Her work in the SHC Data Science team focuses on developing and implementing methods to assess and identify high value applications of machine learning in healthcare settings.
Alison completed her PhD in the Department of Biology at Carleton University in Ottawa, Canada. Her doctoral research focused on developing HyQue, a framework for representing and evaluating scientific hypotheses, and applying this framework to discover genes related to aging. She was also a developer for Bio2RDF, an open-source project to build and provide the largest network of Linked Data for the life sciences. Her postdoctoral work at Stanford applied methodologies developed during her PhD to study spinal cord injury in model organisms and humans in a collaboration with scientists at the University of Miami.
Alison discusses her career journey, how OHDSI impacts her research at Stanford, critical knowledge gaps that can be addressed by the Perinatal and Reproductive Health workgroup, and more in the latest edition of the Collaborator Spotlight.
Community Leaders Host OHDSI RWE Revolution: Igniting Data Modernization with Harmonized Standards for Cutting-Edge Health Research at AMIA 2023
 Several community leaders collaborated to highlight the power and potential of OHDSI during the opening day of the 2023 Symposium. Atif Adam, Asieh Golozar, Ben Martin, Paul Nagy, Gowtham Rao, Christian Reich, Mui Van Zandt, and Ross Williams led a full day workshop entitled Igniting Data Modernization with Harmonized Standards for Cutting-Edge Health Research. You can find those slides here.
Several community leaders collaborated to highlight the power and potential of OHDSI during the opening day of the 2023 Symposium. Atif Adam, Asieh Golozar, Ben Martin, Paul Nagy, Gowtham Rao, Christian Reich, Mui Van Zandt, and Ross Williams led a full day workshop entitled Igniting Data Modernization with Harmonized Standards for Cutting-Edge Health Research. You can find those slides here.
There were sessions focused on standardization, the value of reusable definitions of disease for research, the collaborative open-science community, building concept sets, several open-source tools like Strategus and ATLAS, evidence at scale, and more. There were presentations, panels and hands-on demonstrations.
This session was not recorded, but the slides can be accessed above.
Collaborator Showcase: Atif Adam
 Dr. Atif Adam is a systems scientist and researcher boasting over a decade of diversified experience spanning academia, industry, and entrepreneurial ventures. He attained his doctorate in Health Systems Science and Computational Epidemiology. In addition, Dr. Adam completed his clinical training in Internal Medicine and secured master’s degrees in Health Policy and Spatial Epidemiology.
Dr. Atif Adam is a systems scientist and researcher boasting over a decade of diversified experience spanning academia, industry, and entrepreneurial ventures. He attained his doctorate in Health Systems Science and Computational Epidemiology. In addition, Dr. Adam completed his clinical training in Internal Medicine and secured master’s degrees in Health Policy and Spatial Epidemiology.
His research probes the nuanced relationships between chronic cardiometabolic diseases, mental health, cognitive aging, and health disparities. During his academic appointments at institutions such as Johns Hopkins and Harvard, Dr. Adam pioneered innovative simulation frameworks for cardiometabolic diseases and rigorously evaluated care pathways for the most vulnerable populations. To this end, he employs advanced statistical, geospatial, and systems modeling methodologies. Transitioning into the digital health space, Dr. Adam co-founded and assumed the role of Chief R&D Officer for the digital mental health startup, Rose Health. In this capacity, he harnessed large-scale data and sensor-based models to curate evidence-based digital solutions, primed for proactive patient monitoring.
In his present role as the Associate Director of Epidemiology at IQVIA, Dr. Adam channels his expertise to spearhead transformative real-world evidence (RWE) initiatives. Within the OMOP team at IQVIA, he merges his deep understanding of health systems, an unwavering commitment to health equity, and knowledge in data science to develop and deliver robust RWE studies at scale. Beyond mere discovery, Dr. Adam is ardently devoted to mentorship, nurturing, and guiding the forthcoming generation of health scientists towards a more informed and equitable healthcare horizon. He discusses his career journey, challenges in health equity and how OHDSI is dealing with them, advice for newcomers in OHDSI, and plenty more in the latest Collaborator Spotlight.
2023 Titan Award Honorees Announced At Global Symposium
To recognize OHDSI collaborators (or collaborating institutions) for their contributions towards OHDSI’s mission, the OHDSI Titan Awards are awarded each year at the Global Symposium. We congratulate the eight honorees who were celebrated at OHDSI2023!
The Titan Awards were first introduced in 2018, so this is the sixth class of honorees. 92 individuals or teams were nominated for Titans by fellow community members this year, and all nominees can be seen on the Titan Awards homepage.
Data Standards: Gowtham Rao and Azza Shoaibi
Methodological Research: Jiayi (Jessie) Tong
Open-Source Development: Katy Sadowski
Clinical Applications: Center for Surgical Science
Community Leadership: Nicole Pratt
Community Collaboration: Cynthia Sung
Community Support: Gyeol Song
OHDSI Symposium Welcomes Largest Attendance, Focuses On Large-Scale Evidence Generation & Collaboration Opportunities
The 2023 OHDSI Global Symposium brought together more than 430 community members from around the world for a three-day event filled with opportunities to learn, connect and forge new relationships.
The main conference was held during Day 1, and featured a plenary on improving the reliability and scale of case validation, a State of the Community presentation by several leaders in the community, and a panel on lessons learned from OHDSI network studies. The collaborator showcase included a record number of posters, software demos and lightning talks, and the closing included a interactive session on large-scale collaboration, escape-room style.
There were also two days of workshops, workgroup meetings and an Introduction to OHDSI tutorial. Videos from all presentations and the tutorial will be posted to the symposium homepage when available. Thank you to those who both volunteered their time to make the event a success or joined us to help push forward OHDSI’s mission of improving health by empowering a community to collaboratively generate the evidence that promotes better health decisions and better care.
Less Than Three Weeks Remain To Global Symposium; Registration Remains Open, But Spots Are Filling Up
We are officially in Global Symposium month! The 2023 OHDSI Global Symposium will be held Oct. 20-22 at the Hilton East Brunswick Hotel & Executive Meeting Center in East Brunswick, N.J., USA.
The agenda for the 2023 Global Symposium main conference is now available and it highlights the most diverse agenda in our event history. It lists the full schedule for all three days, descriptions on the various weekend activities, and all 137 posters and 24 software demos that will be presented during the collaborator showcase. The weekend activities include the full-day HowOften Characterization workshop, and Introduction to OHDSI tutorial, workgroup activities, an HL7 FHIR-OMOP Connectathon, and plenty more.
Registration is still open, but spots are filling up fast. If you have not registered yet, please do so now!
Titan Award Nominees Announced; Winners Will Be Announced During Global Symposium Closing
 Congratulations to the 93 individuals/teams who were nominated for a 2023 Titan Award by a member of the OHDSI community!
Congratulations to the 93 individuals/teams who were nominated for a 2023 Titan Award by a member of the OHDSI community!
Annually, community members are invited to nominate individuals or institutions they feel have made significant contributions towards advancing OHDSI’s mission, vision and values. Once nominations are submitted, the OHDSI Titan Award Committee will select the award winners. Award winners will be announced before the networking reception at the annual symposium.
The award categories, as well as all previous recipients, can be found on the home page. The winners will be announced during the closing ceremony of the 2023 Global Symposium.
Collaborator Spotlight: Jody-Ann McLeggon 
Jody-Ann McLeggon is a program manager at the Columbia University Irving Medical Center. She focuses specifically on the collaboration between OHDSI and the U.S. Food & Drug Administration CBER Biologics Effectiveness and Safety (BEST) Community Engagement and Development Initiative. She also took a leadership role in leading the global community on the development and execution of four network studies in the 2023 Save Our Sisyphus Challenge.
Jody-Ann spent more than five years at Northwell Health as a research supervisor and coordinator. She managed project portfolios for multisite research projects and oversaw research activities and development within the department. She also managed the implementation of NIH studies, clinical trials, and investigator-initiated studies.
She discusses the collaboration between OHDSI and the FDA, how OHDSI can assist regulator agencies in the active surveillance of vaccines and drugs, lessons she learned about network studies, and more in the latest edition of the Collaborator Spotlight.
Please Help Refresh Our OMOP CDM Database; Fill Out Brief Survey By Sept. 8 To Ensure Your Place In The Upcoming Our Journey Annual Report
 As an open source community that wants to share our science with the world, it can be challenging to keep track of the databases around the globe that are currently standardizing their data to the OMOP CDM. The latest version of the OMOP CDM survey is our attempt to do just that. There are just 6 short questions that will help OHDSI understand the range and reach of the OMOP CDM. You should fill this out if you:
As an open source community that wants to share our science with the world, it can be challenging to keep track of the databases around the globe that are currently standardizing their data to the OMOP CDM. The latest version of the OMOP CDM survey is our attempt to do just that. There are just 6 short questions that will help OHDSI understand the range and reach of the OMOP CDM. You should fill this out if you:
- Currently work for an organization whose data has been standardized to the OMOP CDM
- Currently license a database that has been standardized to the OMOP CDM
- Are a member of an organization that sends data to a network that utilizes the OMOP CDM (AllOfUs, N3C)
- Are a member of a federated network that uses the OMOP CDM (EHDEN, DARWIN)
Please fill this survey out by Sept. 8 to ensure your place in the next version of the Our Journey annual report, which will be shared at the OHDSI Global Symposium.
August 2023 Vocabulary Release Includes Seven Refreshes/Updates; 11 Community Contributions Submitted As Part Of New Vocabulary Roadmap Plan
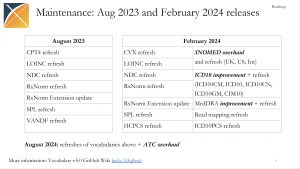 The August 2023 OHDSI Standardized Vocabulary Release was announced during the Aug. 29 community call, and it included a discussion on the seven vocabularies (CPT4, LOINC, NDC, RxNorm, RxNorm Extension, SPL and VANDF) that are either being refreshed or updated. There was also a look ahead to the February 2024 update, which is planned to include work on SNOMED and ICD10, among others.
The August 2023 OHDSI Standardized Vocabulary Release was announced during the Aug. 29 community call, and it included a discussion on the seven vocabularies (CPT4, LOINC, NDC, RxNorm, RxNorm Extension, SPL and VANDF) that are either being refreshed or updated. There was also a look ahead to the February 2024 update, which is planned to include work on SNOMED and ICD10, among others.
Standardized vocabularies was one of three primary focuses for the OHDSI community in 2023, and the development of a community-empowered roadmap for consistent updates has been an early success. The community offered 11 contributions since this plan was developed, and four were incorporated into the current release.
You can learn more about the vocabulary release plan, what vocabularies are still being supported, and how you can provide contributions to the effort by visiting the GitHub page.
Collaborator Spotlight: Davera Gabriel
Davera Gabriel, the Director for Terminology Management at the Johns Hopkins University School of Medicine, has had a distinguished career as a nurse informatician supporting local, regional, and national multi-site implementations of information technology, terminologies, and data standards in human and population health research. She has worked with numerous teams with various clinical and/or technical backgrounds and helped them achieve new heights in informatics.
As an inaugural chair of the HL7 Terminology Services Management Group, co-lead the OMOP + FHIR Working Group, and early participant in the National COVID Cohort Collaborative (N3C), Davera is focused on ways to strengthen the partnership between OHDSI and HL7/FHIR, including through a full-day event at the 2023 Global Symposium.
In the latest edition of the Collaborator Spotlight, Davera talks about her career journey, why OMOP is an ideal partner for the FHIR community, lessons learned as part of the N3C community, the HL7 FHIR-OMOP Connectathon, and plenty more.
Europe Symposium Highlights Community Growth With 90+ Research Posters/Demos, Welcomes Newcomers
 The 2023 Europe Symposium, held July 1-3 in Rotterdam, featured the widest breadth of research ever shared at the European event, and it also hosted several plenary talks and national updates to show how strong the community continues to grow.
The 2023 Europe Symposium, held July 1-3 in Rotterdam, featured the widest breadth of research ever shared at the European event, and it also hosted several plenary talks and national updates to show how strong the community continues to grow.
The main conference included several talks, including sessions focused on European Initiatives Using the OMOP CDM and Data Analysis and Real World Interrogation Network (DARWIN EU®), as well as 10 lightning talks focused on OHDSI Community Evidence. Videos of all talks will be posted on the Europe Symposium homepage when available.
The event welcomed a record number of submissions to the Collaborator Spotlight, including 45 posters on observational data standards and management, 28 on clinical applications, 11 on methods research and five on open-source analytics development. The full list of titles and most of the actual posters can be found on the Europe Showcase homepage. A full recap of the event was shared during the July 11 community call; you can watch that presentation here.
Collaborator Spotlight: Alexander Davydov
 Alexander Davydov has worked as the Technical Team Lead for the Medical Vocabulary team in Odysseus Data Services for more than five years. Alex and his team play an essential role in supporting the OMOP Standardized Vocabulary development for OHDSI, as well as the vocabulary needs in the OMOP CDM ETL-project-related mapping activities. He helped develop the recently completed OHDSI Vocabularies Landscape Assessment and plan the future roadmap for releases and community contributions.
Alexander Davydov has worked as the Technical Team Lead for the Medical Vocabulary team in Odysseus Data Services for more than five years. Alex and his team play an essential role in supporting the OMOP Standardized Vocabulary development for OHDSI, as well as the vocabulary needs in the OMOP CDM ETL-project-related mapping activities. He helped develop the recently completed OHDSI Vocabularies Landscape Assessment and plan the future roadmap for releases and community contributions.
Before joining OHDSI in 2018, he worked as a clinical microbiologist and a lecturer in academia. He is a fully registered physician and obtained his Medical Doctor degree as well as his Doctor of Philosophy training from Belarusian State Medical University, Minsk, Belarus. Alex has collected extensive experience in the OMOP CDM conventions, particularly around the proper use of the vocabularies. Alex contributed to the CDM/Vocabulary, Vaccine vocabulary, Registry (formerly UK Biobank), ATC, and Oncology working groups.
In the latest collaborator spotlight, Alex shares how his versatile background has benefitted him in all areas of OHDSI, when and why he developed his passion for the community, and his vision for OHDSI’s standardized vocabularies.
OHDSI Standardized Vocabulary Assessment, Future Roadmap & Community Contributions Highlighted
 The June 6 OHDSI Community Call featured a session focused on OHDSI Standardized Vocabularies: Landscape, Roadmap & Community Contributions. Following the release of the OHDSI Standardized Vocabularies Assessment, leaders from the vocabulary team presented findings and next steps, including ways to create a more transparent and reliable release cycle.
The June 6 OHDSI Community Call featured a session focused on OHDSI Standardized Vocabularies: Landscape, Roadmap & Community Contributions. Following the release of the OHDSI Standardized Vocabularies Assessment, leaders from the vocabulary team presented findings and next steps, including ways to create a more transparent and reliable release cycle.
This session was led by:
• Anna Ostropolets (Director, Head of the Innovation Lab, Odysseus Data Services, Inc.)
• Alexander Davydov (Technical Team Lead, Odysseus Data Services, Inc.)
• Christian Reich (Senior Researcher, Erasmus University Medical Center; Professor of Practice, Northeastern University)
Both the full presentation and the landscape assessment are available.
Collaborator Spotlight: Mengling ‘Mornin’ Feng
 Mengling ‘Mornin’ Feng is an Assistant Professor at the National University of Singapore and the Assistant Director of Research (Healthcare) at the Institute of Data Science. He has several research interests, including artificial intelligence solutions for healthcare challenges, causal inference for evidence-based medicine, and deep learning models for medical image analysis.
Mengling ‘Mornin’ Feng is an Assistant Professor at the National University of Singapore and the Assistant Director of Research (Healthcare) at the Institute of Data Science. He has several research interests, including artificial intelligence solutions for healthcare challenges, causal inference for evidence-based medicine, and deep learning models for medical image analysis.
Mornin has been an OHDSI leader in helping to spread the community to the Asia-Pacific region. He is the chair of the Singapore Chapter, and recently helped develop a collaboration with the Singapore Ministry of Health that has led the Ministry to use the OMOP CDM as the main platform for research.
In the latest edition of the Collaborator Spotlight, Mornin discusses his professional journey, how he connected with OHDSI, recent developments within the Singapore and APAC regions, and plenty more.
 OHDSI 2023 Collaborator Showcase Deadline Is June 16, 8 pm EST; Showcase Will Be Expanded Throughout Symposium Weekend
OHDSI 2023 Collaborator Showcase Deadline Is June 16, 8 pm EST; Showcase Will Be Expanded Throughout Symposium Weekend
After receiving a record number of Collaborator Showcase submissions for the 2022 Global Symposium, as well as for both the 2023 European and Asia-Pacific (APAC) Symposiums, the 2023 Global Symposium will host extended hours for the upcoming Collaborator Showcase, including sessions during both weekend days.
The submission deadline for the 2023 Collaborator Showcase is approaching, however. All brief reports must be submitted by Friday, June 16, at 8 pm EST. Please use this link to send in your submissions. More details about the collaborator showcase are available here.
The 2023 Global Symposium will be held Oct. 20-22 in East Brunswick, New Jersey, USA, at the Hilton East Brunswick Hotel & Executive Meeting Center. Registration is now open for the event, and more details, including hotel room block information, is available on the OHDSI2023 homepage.
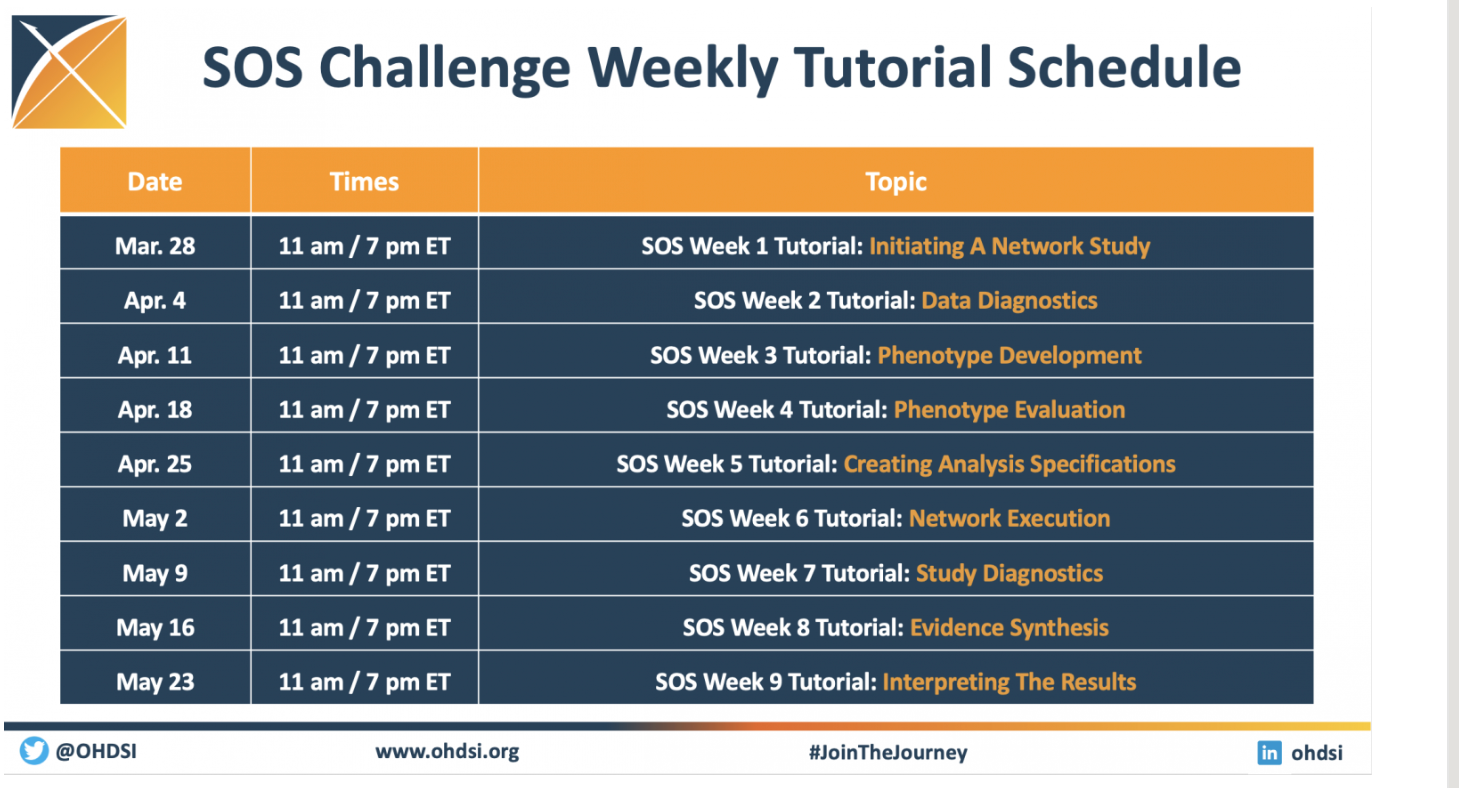
SOS Challenge Update: Network Execution Tutorials Highlight Week 6 Of Challenge; Study Diagnostics Sessions Set For May 9
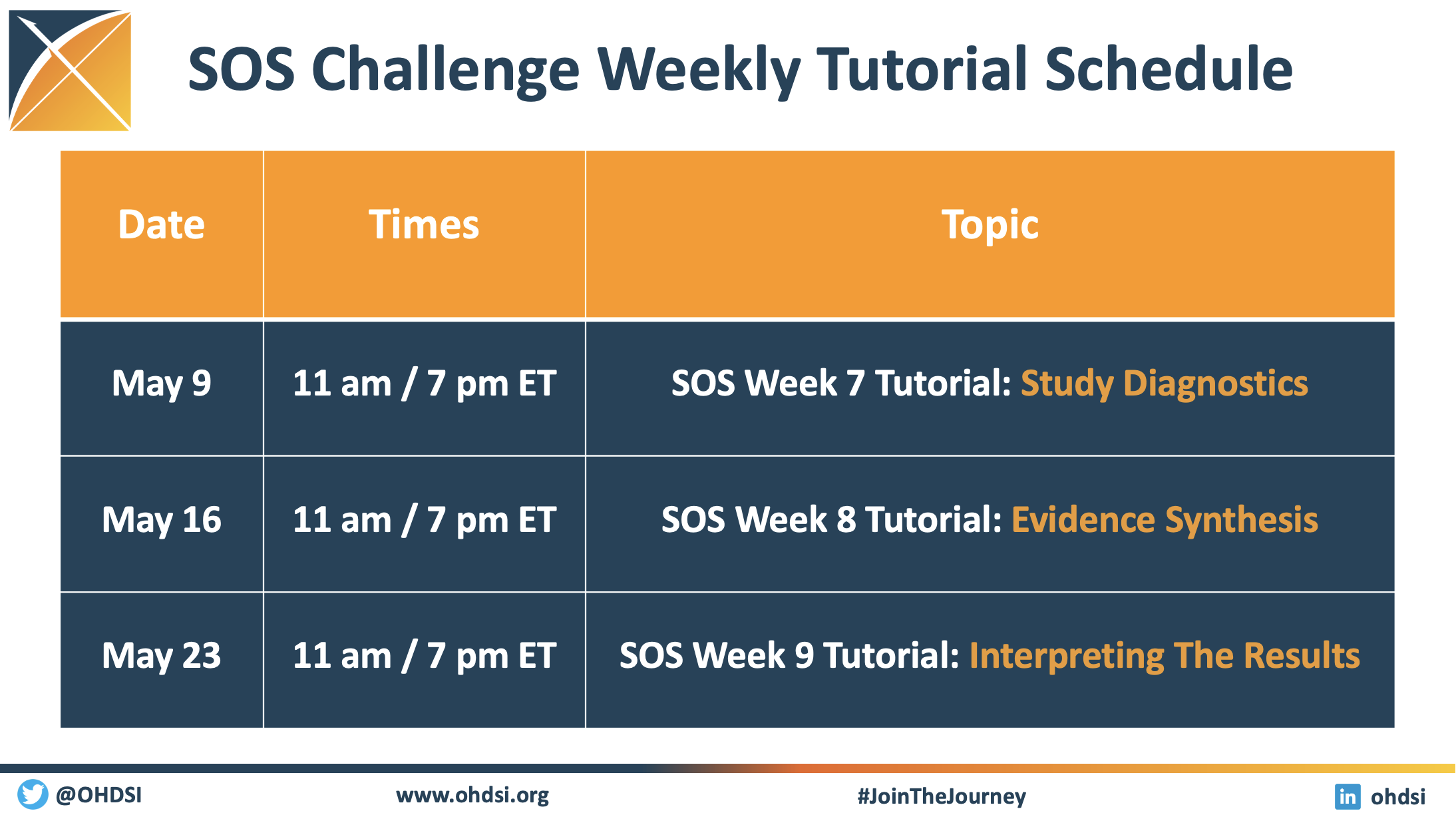 The Save Our Sisyphus (SOS) Challenge is entering Week 7, and the global activity on these network studies continues to inspire. We are collaborating simultaneously on four studies, each of which will be designed, implemented, executed and disseminated by members of the OHDSI global community.
The Save Our Sisyphus (SOS) Challenge is entering Week 7, and the global activity on these network studies continues to inspire. We are collaborating simultaneously on four studies, each of which will be designed, implemented, executed and disseminated by members of the OHDSI global community.
As you can see on the graphic, there are three weeks of tutorials remaining, including two sessions on May 9 that focus on study diagnostics. The first one, held during the traditional OHDSI community call, will be led by George Hripcsak and Fan Bu.
The community has led six weeks of tutorials already, highlighting both the early and middle steps involved in running or participating in a network study. Please visit our SOS Challenge homepage to watch any of these sessions, or to get important links to participate in any of the ongoing studies.
Collaborator Spotlight: Azza Shoaibi
 Azza Shoaibi has been collaborating with the OHDSI community for several years, and has recently taken up new leadership opportunities around phenotyping.
Azza Shoaibi has been collaborating with the OHDSI community for several years, and has recently taken up new leadership opportunities around phenotyping.
An Associate Director with Janssen Research and Development, Inc., Shoaibi first found OHDSI while she was a postdoctorate fellow in bioinformatics at the Medical University of South Carolina. Since then she has led or participated in several network studies, helped lead the first two Phenotype Phebruaries, and presented during the lightning talks at the 2022 OHDSI Global Symposium.
In the latest collaborator spotlight, Azza discusses her journey to observational health data work, why the science of phenotyping is both challenging and exciting, why she serves on the OHDSI Symposium Scientific Review Committee, and plenty more!
DevCon 2023 Introduces New Possibilities, Opportunities Within Open-Source Community, Opens Applications For 2023-24 Cohort
 The Open-Source Community hosted DevCon 2023 as a way to both welcome and inform both new and veteran developers in the OHDSI Community. Organized by Paul Nagy and Adam Black, the event featured 11 short talks around five topics (see agenda) in the first session, followed by a trio of workshops that focused on significant topics for sustained success within our open-source community.
The Open-Source Community hosted DevCon 2023 as a way to both welcome and inform both new and veteran developers in the OHDSI Community. Organized by Paul Nagy and Adam Black, the event featured 11 short talks around five topics (see agenda) in the first session, followed by a trio of workshops that focused on significant topics for sustained success within our open-source community.
The event also opened the application process for the Khieron Contributor 2023-24 Cohort. This program will help onboard and mentor open-source developers in the community for a second straight year. Katy Sadowski, a member of the original cohort, provided a video and written testimonial about her experience with the Kheiron Cohort.
You can apply now for this cohort using this link; all applications are due May 15.
Registration Opens For All Three 2023 Symposiums; Call For Participation In Both APAC, Europe Will Conclude In April
 The 2023 OHDSI Global Symposium will be held Oct. 20-22 at the NEW location of the Hilton East Brunswick Hotel & Executive Meeting Center in East Brunswick, N.J. Registration has opened, and the call for collaboration is open until Friday, June 16, at 8 pm ET. Check out the symposium homepage for more information and updates as we get closer to the OHDSI symposium.
The 2023 OHDSI Global Symposium will be held Oct. 20-22 at the NEW location of the Hilton East Brunswick Hotel & Executive Meeting Center in East Brunswick, N.J. Registration has opened, and the call for collaboration is open until Friday, June 16, at 8 pm ET. Check out the symposium homepage for more information and updates as we get closer to the OHDSI symposium.
The 2023 European Symposium — titled “Full Steam Ahead” — will be held July 1-3 in Rotterdam. The main conference will be held Monday, July 3, while there will be tutorials on the weekend of July 1-2. Registration and the call for participation has opened for this event. The deadline for abstracts is April 30.
The 2023 Asia-Pacific (APAC) Symposium will be held July 13-14 at the University of New South Wales in Sydney, Australia, and it will follow MedInfo 2023 (July 8-12 in Sydney, Australia). Registration and the call for participation has opened for this event. The call for participation deadline for abstracts is April 15. The agenda is now posted on the event homepage. The main conference will be held July 13 with sessions focused on OHDSI Global, Research, and OHDSI APAC, while there will be tutorials held on July 14.
SOS Challenge Focuses On Answering Four Clinical Questions, Learning All Aspects Of Running Or Participating In A Network Study
Collaborator Spotlight: Aniek Markus
SOS Challenge Research Ideas Posted; Vote For A Featured Study, And Share How You Can Be Involved In This Collaborative Activity
 The “Save Our Sisyphus” Network Study Challenge will be a community activity this spring that brings our global collaborators together to lead a single study from idea to publication. This eight-week event will feature weekly tutorials on the Tuesday community calls to demonstrate the best practices developed within OHDSI to generate robust and reliable evidence.
The “Save Our Sisyphus” Network Study Challenge will be a community activity this spring that brings our global collaborators together to lead a single study from idea to publication. This eight-week event will feature weekly tutorials on the Tuesday community calls to demonstrate the best practices developed within OHDSI to generate robust and reliable evidence.
The OHDSI community will provide support through every step of the process, including designing an appropriate protocol, implementing a network analysis package, executing across OHDSI data partners, and preparing a manuscript for publication. The goal is to collaboratively complete this network study over the course of 8 weeks between late March and May, using the open-source tools and process that OHDSI has established.
In order to do the research, though, we must have a question to study. Thank you to everybody in the community who shared their suggested research ideas; four were selected for presentation on the March 7 community call, and now we ask the community to vote for one to be highlighted each week on the community call. We believe all four are terrific ideas, and we hope all four can be completed this spring, but we will focus on one during the community calls.
All four presentations and slides are available here. Please check them out and then vote for one to be featured during the spring.
Phenotype Phebruary 2023 Is Complete, But The Work Continues; Links To All Phenotypes, Discussions and Videos Are Available Here
 “Phenotype Phebruary” is a community-wide initiative to both develop and evaluate phenotypes for health outcomes that could be investigated by the community.
“Phenotype Phebruary” is a community-wide initiative to both develop and evaluate phenotypes for health outcomes that could be investigated by the community.
This is the second year of Phenotype Phebruary in the OHDSI community (look back at Year 1 here). It was introduced during the Jan. 31 community call (watch here), and will go on throughout the month. This year, the leadership team of Gowtham Rao and Azza Shoaibi helped identify 10 phenotypes that are being investigated throughout the month. If you would like to join the discussions around any of the phenotypes, please visit our 2023 homepage, which will take you to the proper threads on the OHDSI forums.
Among the links on the homepage are direct links to the phenotypes being investigated, four different discussions around the value and challenges of phenotyping, and videos of both community updates and detailed discussions within the Phenotype Evaluation & Workgroup team on specific phenotypes being investigated.
Save Our Sisyphus Network Study Challenge Set For Spring 2023; Research Ideas To Drive Challenge Sought By Feb. 28
 The “Save Our Sisyphus” Network Study Challenge will be a community activity this spring that brings our global collaborators together to lead a single study from idea to publication. The OHDSI community will provide support through every step of the process, working with you to design an appropriate protocol, implement a network analysis package, execute across OHDSI data partners, and prepare a manuscript for publication. Our goal is to collaboratively complete this network study over the course of 8 weeks across April and May, using the open-source tools and process that OHDSI has established.
The “Save Our Sisyphus” Network Study Challenge will be a community activity this spring that brings our global collaborators together to lead a single study from idea to publication. The OHDSI community will provide support through every step of the process, working with you to design an appropriate protocol, implement a network analysis package, execute across OHDSI data partners, and prepare a manuscript for publication. Our goal is to collaboratively complete this network study over the course of 8 weeks across April and May, using the open-source tools and process that OHDSI has established.
Our 2023 SOS Challenge can focus on any of the three analysis use cases that we regularly discuss in OHDSI: clinical characterization-descriptive statistics for disease natural history and treatment utilization; population-level effect estimation-causal inference for safety surveillance and comparative effectiveness; or, patient-level prediction-machine learning for disease interception and precision medicine.
We are particularly interested in supporting junior researchers looking to conduct their first network analysis, but all OHDSI collaborators are welcome and encouraged to submit their ideas. If you are interested in participating in the SOS Challenge, please complete this form, where you’ll be asked to provide your research question and a statement about why you think your question is clinically important and should be answered by the OHDSI network.
Vocabulary Landscape Assessment Will Inform OHDSI Prioritization; Please Complete Survey(s) By Feb. 23
 Anna Ostropolets introduced a vocabulary landscape assessment survey to directly inform which vocabularies and activities the vocabulary team prioritizes in 2023. The feedback on the vocabularies’ use and problems will directly inform which vocabularies and activities are prioritized in 2023.
Anna Ostropolets introduced a vocabulary landscape assessment survey to directly inform which vocabularies and activities the vocabulary team prioritizes in 2023. The feedback on the vocabularies’ use and problems will directly inform which vocabularies and activities are prioritized in 2023.
Filling the forms should take about 10 minutes. We are seeking information about the vocabularies you use, what you think about their quality, challenges you encountered and what we can do to improve the content and the process of distribution and update.
There are TWO surveys that can be filled out. The first is an overall form for people who use the vocabularies for research, ETL, software development or something else. The second is an ETL-specific form for those who perform ETL or have an OMOP CDM instance at their disposal. Please fill it for each data source you have as we will use this information to prioritize the vocabularies we work on.
Video: Where Can OHDSI Go Together In 2023
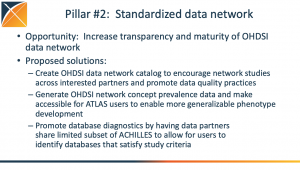
 During the initial OHDSI community call of 2023, Patrick Ryan highlighted several community initiatives, activities and opportunities that can provide a foundation to further our shared mission of improving health by empowering a community to collaboratively generate the evidence that promotes better health decisions and better care. There were three main pillars around OHDSI work in 2023 that was highlighted: 1) standardized vocabularies; 2) a
During the initial OHDSI community call of 2023, Patrick Ryan highlighted several community initiatives, activities and opportunities that can provide a foundation to further our shared mission of improving health by empowering a community to collaboratively generate the evidence that promotes better health decisions and better care. There were three main pillars around OHDSI work in 2023 that was highlighted: 1) standardized vocabularies; 2) a 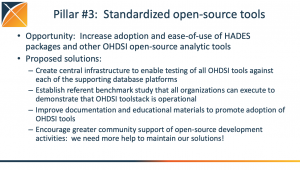 standardized data network; 3) standardized open-source tools. There was also discussion around a community-run network study in the spring, the return of Phenotype Phebruary and DevCon, and plenty more.
standardized data network; 3) standardized open-source tools. There was also discussion around a community-run network study in the spring, the return of Phenotype Phebruary and DevCon, and plenty more.
The full presentation is available here. During the Jan. 24 community call, Patrick will join Anna Ostropolets and Clair Blacketer to discuss specific ways in which the community can collaborate to address several of the priorities mentioned in this session.
New PLP Demos Highlight New Options in v6, Strategus Possibilities
 PatientLevelPrediction, a part of the HADES open-source tool library, is an R package for building and validating patient-level predictive models using data in the OMOP Common Data Model format. Check out the PatientLevelPrediction (PLP) github page for more information.
PatientLevelPrediction, a part of the HADES open-source tool library, is an R package for building and validating patient-level predictive models using data in the OMOP Common Data Model format. Check out the PatientLevelPrediction (PLP) github page for more information.
PLP workgroup co-lead and package maintainer Jenna Reps created a series of demo videos to provide assistance with using v6 of the package. You can check out the descriptions and videos here, or on our OHDSI YouTube page (check out the tutorials playlist).
Specific video topics include how to extract data and develop a single model, how to design prediction models and develop multiple models, how to use the PLP v6 shiny app, how to use the new OHDSI R package Strategus and OHDSI modules to develop an OHDSI prediction development network study, and how to run an OHDSI prediction network study using the new Strategus approach.
 Video: 2022 OHDSI In Review
Video: 2022 OHDSI In Review
Patrick Ryan presented a comprehensive look back at the activities, publications, open-source developments and more from the OHDSI community throughout 2022 during a December community call. This month-by-month journey highlights community OKRs from the beginning of the year, activities like Phenotype Phebruary and DevCon, as well as our first three in-person events since the COVID pandemic, the European, Global and APAC Symposia.
The full video presentation is available here.
 Collaborator Spotlight: Thamer AlShammary
Collaborator Spotlight: Thamer AlShammary
Dr. Thamir Alshammari is an advisor to the President of the Saudi Food and Drug Authority (SFDA). He is a pharmacoepidemiology and pharmacovigilance consultant. He is also a distinguished expert for the Chinese National drug safety and policy center at Xian, China, as well as an ambassador to the British Pharmacological Society. He is a senior researcher at the Medication Safety Research Chair.
Thamir has been an active contributor to the OHDSI community for several years. He collaborates in several workgroups, including Population-Level Estimation, Health Equity and the recently-completed Vaccine Evidence WG, and has been a contributor in several important network studies, which are discussed below. He also brings unique perspective as somebody working with a national government on critical healthcare issues.
Thamir discusses his journey in pharmacovigilance and health policy, and how that path found the OHDSI community, as well as some of the work he focuses on within OHDSI. He also discusses why OHDSI can be a difference maker in generating trustworthy evidence, tools and best practices within the community, and plenty more in the latest edition of the collaborator spotlight.
New Software Tutorials Page Offers Demos In Open-Source Tools Designed To Enhance Community Research
 The open-source tools that empower OHDSI’s global research initiatives are not only available to the community, but they are also developed by the community. Leaders from around the world have developed tools that provide the foundation for OHDSI collaborators to engage in robust, reliable and reproducible observational health research.
The open-source tools that empower OHDSI’s global research initiatives are not only available to the community, but they are also developed by the community. Leaders from around the world have developed tools that provide the foundation for OHDSI collaborators to engage in robust, reliable and reproducible observational health research.
During various OHDSI community calls, developers join and provide “10-minute tutorials” to educate the community about the tool’s potential impact, and how they can be used in research. These tutorials are available on the OHDSI YouTube channel, but are also posted on the new open-source tutorials page.
To learn more about HADES, a set of open source R packages for large scale analytics, including population characterization, population-level causal effect estimation, and patient-level prediction, check out the GitHub page.
OHDSI Tutorial Provides An Introductory Journey From Data To Evidence (Videos Now Available)
2022 Symposium Connects 400+ Collaborators For Weekend Of Sharing & Learning Under Theme Of “Building A Healthier World Together”
 More than 400 community members from around the world connected Oct. 14-16 in Bethesda, Md., for the 2022 OHDSI Symposium, the first in-person global symposium since 2019. The weekend theme was “Building A Healthier World Together,” and both the main conference and the weekend activities, including a full-day tutorial, highlighted the different ways OHDSI has impacted global healthcare, and the steps needed to be taken to build on that foundation.
More than 400 community members from around the world connected Oct. 14-16 in Bethesda, Md., for the 2022 OHDSI Symposium, the first in-person global symposium since 2019. The weekend theme was “Building A Healthier World Together,” and both the main conference and the weekend activities, including a full-day tutorial, highlighted the different ways OHDSI has impacted global healthcare, and the steps needed to be taken to build on that foundation.
The main day featured a plenary presentation on Objective Diagnostics: A pathway to provably reliable evidence, as well as talks on OHDSI support for regulatory authorities. A record-setting amount of submissions for the collaborator showcase led to two hours of poster and demo presentations, as well as eight lightning talks. There was also a state of the community talk at the beginning and a closing talk that discussed how OHDSI can move forward together, how collaboration can take place globally and … legos.
The OHDSI 2022 Symposium web page includes all the videos and slides from the weekend, including our “Introductory Journey from Data to Evidence” tutorial, so please visit the page and check out everything that happened during the symposium weekend.
Titan Award Winners Announced at 2022 Symposium
 The 2022 Titan Award winners were announced during the closing of the 2022 OHDSI Symposium. Congratulations to all of our winners, and to the 50+ individuals or groups who were nominated by members of our community.
The 2022 Titan Award winners were announced during the closing of the 2022 OHDSI Symposium. Congratulations to all of our winners, and to the 50+ individuals or groups who were nominated by members of our community.
Data Standards: Melanie Philofsky, Odysseus Date Services
Methodological Research: Fan Bu, UCLA
Open-Source Development: Egill Fridgeirsson, Erasmus MC and James Gilbert, Janssen Research and Development
Clinical Applications: Xintong Li, University of Oxford
Community Collaboration: Ajit Londhe, Boehringer Ingelhei
Community Leadership: Paul Nagy, Johns Hopkins University
Community Support: Craig Sachson, Columbia University
George Hripcsak, Chair Of OHDSI Coordinating Center, to be Awarded Morris F. Collen Award of Excellence at AMIA 2022 Symposium
 The American College of Medical Informatics (ACMI) will present the 2022 Morris F. Collen Award of Excellence to George Hripcsak, MD, MS, FACMI, Vivian Beaumont Allen Professor and Chair of the Department of Biomedical Informatics, Columbia University, during the opening session of the American Medical Informatics Association (AMIA) 2022 Annual Symposium. AMIA’s Annual Symposium is November 5-9 in Washington, D.C.
The American College of Medical Informatics (ACMI) will present the 2022 Morris F. Collen Award of Excellence to George Hripcsak, MD, MS, FACMI, Vivian Beaumont Allen Professor and Chair of the Department of Biomedical Informatics, Columbia University, during the opening session of the American Medical Informatics Association (AMIA) 2022 Annual Symposium. AMIA’s Annual Symposium is November 5-9 in Washington, D.C.
In honor of Morris F. Collen, a thought leader in the field of medical informatics, this prestigious award is presented to an individual whose personal commitment and dedication to medical informatics has made a lasting impression on the field. The award is determined by ACMI’s Awards Committee.
“ACMI is pleased to recognize Dr. Hripcsak for his substantial accomplishments to the field of biomedical informatics,” said ACMI President Genevieve Melton-Meaux, MD, PhD, FACMI, Professor of Surgery and Health Informatics and Director of the Center for Learning Health System Sciences, University of Minnesota, Chief Analytics and Care Innovation Officer, Fairview Health Services. “Dr. Hripcsak’s contributions have stretched around the globe and his collaboration with those both inside and outside of our field has expanded the reach and impact of informatics. As a mentor, Dr. Hripcsak reminds his peers to ‘Do good work.’ I am grateful for the guidance and mentorship he has provided to me and many others in biomedical informatics.”
Collaborator Spotlight: Jing Li
 Jing Li is an Associate Director of Data Science at IQVIA, where she is leading a global team of data scientists on real world studies, focusing on treatment patterns, and drug safety studies. She has several years of industry experience in predictive modeling, machine learning, and data management, and she decided to focus her work on healthcare research in 2019.
Jing Li is an Associate Director of Data Science at IQVIA, where she is leading a global team of data scientists on real world studies, focusing on treatment patterns, and drug safety studies. She has several years of industry experience in predictive modeling, machine learning, and data management, and she decided to focus her work on healthcare research in 2019.
Jing has grown into a leader in the OHDSI Asia-Pacific (APAC) Community. She leads the bi-weekly APAC community calls and is part of the steering group for both the APAC workgroup and APAC symposium planning committee. She was also a co-author on the first ever network study published by the APAC workgroup, Analysis of Dual Combination Therapies Used in Treatment of Hypertension in a Multinational Cohort.
In our most recent edition of the Collaborator Spotlight, Jing discusses her career and how she moved into healthcare, her excitement about the growing APAC community, and plenty more.
Perseus Tool Highlighted In Clinical Registry Efforts In OHDSI Presentation
 Perseus, an open-source tool developed within the OHDSI community, combines intuitive and easy to use Web-based UI for design and implement ETL (extract, transform, and load) configuration and service for conversion the native/raw data to the OMOP Common Data Model (CDM). Additionally, Perseus has embedded tools for search in the standardized vocabularies, generates documentation for the ETL process, create the code mappings and data quality check.
Perseus, an open-source tool developed within the OHDSI community, combines intuitive and easy to use Web-based UI for design and implement ETL (extract, transform, and load) configuration and service for conversion the native/raw data to the OMOP Common Data Model (CDM). Additionally, Perseus has embedded tools for search in the standardized vocabularies, generates documentation for the ETL process, create the code mappings and data quality check.
Zachary Wang led a live demo of this tool during the Sept. 13 community call, which was focused on ‘Clinical Registry Efforts in OHDSI’. Paul Nagy, Matthew Robinson and Lee Evans also presented on this important topic, while Emily Pfaff, a leader in the National COVID Cohort Collaborative, will join a future call to discuss Distributed Machine Learning Using OMOP.
Symposium Agenda Posted; Register Now To Be Part Of OHDSI2022!
 The OHDSI Symposium will take place Oct. 14-16 at the Bethesda North Marriott Hotel and Conference Center, and the full weekend agenda has been posted. The main conference day will include a plenary session on Objective Diagnostics: A pathway to provably reliable evidence, presentations on OHDSI support for regulatory authorities, a collaborator showcase that includes 100+ posters, 17 software demos and 8 lightning talks, and a closing talk you won’t want to miss.
The OHDSI Symposium will take place Oct. 14-16 at the Bethesda North Marriott Hotel and Conference Center, and the full weekend agenda has been posted. The main conference day will include a plenary session on Objective Diagnostics: A pathway to provably reliable evidence, presentations on OHDSI support for regulatory authorities, a collaborator showcase that includes 100+ posters, 17 software demos and 8 lightning talks, and a closing talk you won’t want to miss.
The weekend will include a full-day Saturday tutorial on An Introductory Journey From Data To Evidence, as well as several workgroup activities planned throughout the weekend. The agenda includes a full list of the software demos and lightning talks; a future version will include all of the poster titles and presenters.
There will be capped totals for all sessions, so please don’t wait until the last minute to secure your spot for our highlight event of the year!
Collaborator Spotlight: Paul Nagy
 Paul Nagy, PhD, FSIIM is Associate Professor in the Johns Hopkins University School of Medicine Department of Radiology with a joint appointments in Medicine and the Department of Biomedical Engineering in the School of Engineering. He received his BS from Carnegie Mellon University and his PhD at the Medical College of Wisconsin. His research focus is developing biomarkers from medical imaging to enable real world reproducible evidence from observational research.
Paul Nagy, PhD, FSIIM is Associate Professor in the Johns Hopkins University School of Medicine Department of Radiology with a joint appointments in Medicine and the Department of Biomedical Engineering in the School of Engineering. He received his BS from Carnegie Mellon University and his PhD at the Medical College of Wisconsin. His research focus is developing biomarkers from medical imaging to enable real world reproducible evidence from observational research.
He is the director of education for the training programs in the Biomedical Informatics and Data Science section of the Department of Medicine. He leads the Observational Health and Data Science Informatics (OHDSI) efforts at Johns Hopkins as part of the Precision Medicine initiative. He is a leader in the OHDSI community, especially within the developer community. He began the Kheiron Cohort in 2022, which serves to welcome and mentor developers, and he joined Adam Black to start the open-source community workgroup this year as well.
Nagy, who is also active in several other workgroups and co-leads the Medical Imaging WG, helped lead the DevCon 2022 event, and has developed tools to track OHDSI impact in several areas. He shared some thoughts about his career, his many contributions to the OHDSI community, and plenty more in the latest Collaborator Spotlight.
New CDM Update Process Includes New Decision Tree, Clarifies How Data Model Requests Are Made, Codified
 The July 26 community call featured a session led by Clair Blacketer, Paul Nagy and Davera Gabriel on what to do when your data does not fit into the OMOP CDM.
The July 26 community call featured a session led by Clair Blacketer, Paul Nagy and Davera Gabriel on what to do when your data does not fit into the OMOP CDM.
Our community continues to expand globally, and both individuals and organizations often look for new enhancements to the CDM. There will be a new decision tree and process implemented to try and streamline this procedure and to clarify how data model requests are made and codified, and these were presented and discussed during this meeting.
The presentation can be seen here, and it includes specific use cases presented by both Nagy and Gabriel to help show how the decision tree and process happens.
All Presentations From 2022 European Symposium Are Now Available
Collaborator Spotlight: Nicole Pratt
 Nicole Pratt, a longtime collaborator with the OHDSI community, is the Deputy Director of the Quality Use of Medicines and Pharmacy Research Centre at the University of South Australia. She is a member of the Drug Utilisation Subcommittee (DUSC) of the Australian Department of Health Pharmaceutical Benefits Advisory Committee (PBAC).
Nicole Pratt, a longtime collaborator with the OHDSI community, is the Deputy Director of the Quality Use of Medicines and Pharmacy Research Centre at the University of South Australia. She is a member of the Drug Utilisation Subcommittee (DUSC) of the Australian Department of Health Pharmaceutical Benefits Advisory Committee (PBAC).
She has a particular interest in new statistical methodologies to study the effectiveness and safety of medicine use and in the development of tools for post-marketing surveillance of medicines. This interest helped lead her to be a collaborator within the OHDSI LEGEND initiative; the LEGEND hypertension study led to a 2019 Lancet publication that found that the most popular hypertension drug wasn’t the most effective.
Nicole has also been one of the leaders in the growing Asia-Pacific (APAC) OHDSI community and the leader of the Australia chapter. She discusses her own research, the developments in the OHDSI APAC community, the importance of large community initiatives and much more in the latest edition of the Collaborator Spotlight.
Network Study Leads Share Updates, Calls For Collaboration During May 2022 Community Call
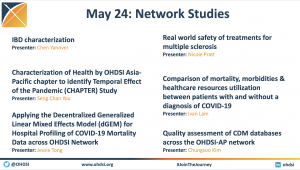 Six OHDSI network studies were presented during a May 2022 community call to both inform the community about global research efforts (either ongoing or recently completed), as well as call for collaborators to join in these important research projects.
Six OHDSI network studies were presented during a May 2022 community call to both inform the community about global research efforts (either ongoing or recently completed), as well as call for collaborators to join in these important research projects.
These brief updates were recorded and posted to the OHDSI YouTube channel, and all presentations are available here. You can also access slides above each of the video presentations.
If you are interested in collaborating, please reach out to the presenter on Teams or within the OHDSI forums.
DARWIN EU Initiative — How OHDSI Can Collaborate With Erasmus MC and the EMA to Assist in Delivering Real-World Evidence & Supporting Regulatory Decision-Making Across Europe
Collaborator Spotlight: Asieh Golozar
 Asieh Golozar, a longtime OHDSI collaborator and the 2021 Titan Award for Clinical Application recipient, is Vice President and Global Head of Data Science at Odysseus Data Services, Inc. She also serves as Professor of the Practice & Director of Clinical Research at the OHDSI Center, Northeastern University.
Asieh Golozar, a longtime OHDSI collaborator and the 2021 Titan Award for Clinical Application recipient, is Vice President and Global Head of Data Science at Odysseus Data Services, Inc. She also serves as Professor of the Practice & Director of Clinical Research at the OHDSI Center, Northeastern University.
Golozar currently leads the Oncology workgroup, and she discusses its impact on her OHDSI journey, as well as its goals for the upcoming year. She also discusses her path to epidemiology (which wasn’t her first career path), why she is working with the Roux Institute around reproducibility, and plenty more in the latest Collaborator Spotlight.
OHDSI Launches Three-Tiered Sponsorship Program To Strengthen Foundation For Global Community Growth
 The OHDSI Coordinating Center, located in the Columbia University Department of Biomedical Informatics, supports the OHDSI open-source community and the shared mission to improve health by empowering a community to collaboratively generate the evidence that promotes better health decisions and better care. Among the ways that the Coordinating Center provides this support is through stewardship of open-community data standards, by enabling open-source development, by facilitating methods research and clinical applications, by encouraging open sharing and evidence dissemination, and by fostering collaborations and empowering a sense of community.
The OHDSI Coordinating Center, located in the Columbia University Department of Biomedical Informatics, supports the OHDSI open-source community and the shared mission to improve health by empowering a community to collaboratively generate the evidence that promotes better health decisions and better care. Among the ways that the Coordinating Center provides this support is through stewardship of open-community data standards, by enabling open-source development, by facilitating methods research and clinical applications, by encouraging open sharing and evidence dissemination, and by fostering collaborations and empowering a sense of community.
OHDSI has introduced a Sponsorship Program, which will allow both corporations and individuals to make meaningful contributions in support of OHDSI’s central coordinating activities. There are three levels of support, including donation amount and benefits to the sponsor, which are detailed on the sponsorship page.
George Hripcsak, Chair and Vivian Beaumont Allen Professor of Biomedical Informatics at Columbia University, provided a detailed presentation on the support provided by the Coordinating Center during an April 2022 community call. That presentation is available here.
Reproducibility In The OHDSI Environment — Why It Matters, And How We Improve Upon It
 The March 29 OHDSI Community Call focused on reproducibility, and three leaders in the community shared presentations on this topic. Anna Ostropolets, a PhD student at Columbia University, shared a presentation on the OHDSI Reproducibility Challenge, a one-day event held during the 2021 OHDSI Symposium that attempted to have teams of OHDSI collaborators reproduce the cohort logic for target, comparator and outcome cohorts for a published study run outside of OHDSI.
The March 29 OHDSI Community Call focused on reproducibility, and three leaders in the community shared presentations on this topic. Anna Ostropolets, a PhD student at Columbia University, shared a presentation on the OHDSI Reproducibility Challenge, a one-day event held during the 2021 OHDSI Symposium that attempted to have teams of OHDSI collaborators reproduce the cohort logic for target, comparator and outcome cohorts for a published study run outside of OHDSI.
Martijn Schuemie, a Research Fellow in epidemiology analytics at Janssen Research and Development, shared a presentation on creating reproducible studies using OHDSI tools and best practices. Topics included the need for analyses to be repeatable, writing studies as a pipeline, and how to create the proper study package.
Asieh Golozar, Vice President and Global Head of Data Science at Odysseus Data Services, Inc., shared a community update on the OHDSI Reproducibility Service, which is centered at The Roux Institute at Northeastern University. This talk includes discussion of what type of study makes for a good candidate for reproducibility, the approach with the Center, and a proof of concept study that is seeking data partner collaboration.
Videos and slides from the presentation are included within the reproducibility session homepage.
OHDSI Hosts DevCon 2022 April 22 To Welcome & Mentor New Contributors To Our Open-Source Environment
 The Open-Source Community is hosting the first Dev Con on Friday, April 22 (8 am – 12 pm ET) as a way of accepting and mentoring new contributors to our environment. Organized by Paul Nagy and Adam Black, the event will include multiple workshops, talks and a panel discussion to both welcome and engage both current and future developers within OHDSI.
The Open-Source Community is hosting the first Dev Con on Friday, April 22 (8 am – 12 pm ET) as a way of accepting and mentoring new contributors to our environment. Organized by Paul Nagy and Adam Black, the event will include multiple workshops, talks and a panel discussion to both welcome and engage both current and future developers within OHDSI.
Who should consider registering for DevCon? We are looking for people who are interested in participating with an OHDSI project team, seeing ‘under the hood’ of the OHDSI engine, or being mentored by professional developers.
The full agenda, including all eight current workshop offerings and the planned panel discussion, as well as registration links are available on our DevCon homepage.
OHDSI2022 Symposium Registration Opens For Friday Main Conference, Saturday Tutorial
 We are thrilled to announce that registration for the 2022 OHDSI Symposium, which will be held Oct. 14-16 at the Bethesda North Marriott Hotel & Conference Center, is now open!
We are thrilled to announce that registration for the 2022 OHDSI Symposium, which will be held Oct. 14-16 at the Bethesda North Marriott Hotel & Conference Center, is now open!
The main conference will be held Friday, Oct. 14, while a full-day tutorial will be held Saturday, Oct. 15. Other community activities, mainly focused on OHDSI workgroups, will be held both Oct. 15 and Oct. 16. The OHDSI2022 homepage has more information, as well as registration links to both the conference and the tutorial (these are separate events and each requires its own registration), information on the collaborator showcase, hotel room blocks, and plenty more.
Direct registration is available for both the main conference and the full-day tutorial. Please continue to check our symposium homepage and our social platforms, and join the weekly OHDSI community calls, for more information.
Standardized Vocabularies Presentation Highlights Development And Community Impact, Looks To Future Growth
 The March 22 OHDSI Community Call provided an in-depth look at the OHDSI vocabulary, from how it is developed, to how it can be utilized, and where it should grow from here. Three leaders from the vocabulary workgroup joined to present a trio of topics for this session (see below), and the video is now available along with the Standardized Vocabulary introduction from the book of OHDSI.
The March 22 OHDSI Community Call provided an in-depth look at the OHDSI vocabulary, from how it is developed, to how it can be utilized, and where it should grow from here. Three leaders from the vocabulary workgroup joined to present a trio of topics for this session (see below), and the video is now available along with the Standardized Vocabulary introduction from the book of OHDSI.
Michael Kallfelz (Physician Executive • Odysseus Data Services) led a discussion on “A peek into the OHDSI vocabulary engine room,” while Patrick Ryan (Vice President, Observational Health Data Analytics • Janssen Research & Development) provided a presentation on “Fun things you can learn with the OHDSI standardized vocabularies.” Christian Reich (Vice President, RWE Systems • IQVIA) concluded the presentation with a forward-looking discussion: “Time for reflection • Where are we? Where should we be?”
This session also introduced as to members of the OHDSI vocabulary team and all the work it takes to create the OHDSI vocabularies, which are critical for the success of our community. The slides from this presentation are available here.
Common Data Model Team Leads CDM 5.4 Workshop; Full Video Tutorial Is Now Available
Collaborator Spotlight: Maxim Moinat
 Maxim Moinat is a data engineer with a demonstrated history of working in bioinformatics and medical informatics. He has worked as a data engineer/software developer for The Hyve since 2016, and recently became a scientific researcher for the Erasmus MC medical informatics department. He has been a long-time collaborator with both the OHDSI and EHDEN communities.
Maxim Moinat is a data engineer with a demonstrated history of working in bioinformatics and medical informatics. He has worked as a data engineer/software developer for The Hyve since 2016, and recently became a scientific researcher for the Erasmus MC medical informatics department. He has been a long-time collaborator with both the OHDSI and EHDEN communities.
Maxim earned a 2021 Titan Award honoree for his invaluable contributions in data standards. He leads the Registry workgroup, contributes to open-source development and has provided tutorials and other presentations during global community calls. He said his passion is to apply his skills to advance biomedical science, ultimately improving healthcare for many patients.
Maxim recently shared several insights in our latest Collaborator Spotlight.
European Medicines Agency Announces OHDSI Collaborator Erasmus University Will Initiate DARWIN EU Coordination Center
 The European Medicines Agency (EMA) announced Feb. 9 that Erasmus University Medical Center Rotterdam has been contracted to establish the DARWIN EU (Data Analysis and Real World Interrogation Network) Coordination Centre.
The European Medicines Agency (EMA) announced Feb. 9 that Erasmus University Medical Center Rotterdam has been contracted to establish the DARWIN EU (Data Analysis and Real World Interrogation Network) Coordination Centre.
The role of the Coordination Centre is to develop and manage a network of real-world healthcare data sources across the EU and to conduct scientific studies requested by medicines regulators and, at a later stage, requested by other stakeholders.
The vision of DARWIN EU is to give EMA and national competent authorities in EU Member States access to valid and trustworthy real-world evidence, for example on diseases, patient populations, and the use, safety and effectiveness of medicines, including vaccines, throughout the lifecycle of a medicinal product.
Peter Rijnbeek, a veteran OHDSI collaborator and Titan Award honoree, is the Chair of the Department of Medical Informatics of the Erasmus Medical Center.
COVER Prediction Model Generated During COVID Study-A-Thon Published; Lead Authors Share Thoughts On Model, Impact
 The first COVID-19 prediction model developed and validated by the OHDSI community following the March 2020 global study-a-thon was recently published by BMC Medical Research Methodology.
The first COVID-19 prediction model developed and validated by the OHDSI community following the March 2020 global study-a-thon was recently published by BMC Medical Research Methodology.
The study “Seek COVER: using a disease proxy to rapidly develop and validate a personalized risk calculator for COVID-19 outcomes in an international network” developed COVID-19 Estimated Risk (COVER) scores that quantify a patient’s risk of hospital admission with pneumonia (COVER-H), hospitalization with pneumonia requiring intensive services or death (COVER-I), or fatality (COVER-F) in the 30-days following COVID-19 diagnosis using historical data from patients with influenza or flu-like symptoms and tested this in COVID-19 patients.
Led by co-first authors Ross Williams and Aniek Markus, both of whom share thoughts on both the model and its impact in this writeup, the team designed a nine-predictor risk model that was validated using more than 44,500 COVID patients (following initial development and validation using more than 6.8 million patients with influenza or flu-like symptoms). This model predicts hospitalization, intensive services, and death, and can help provide reassurance for low-risk patients, while shielding high-risk patients, as many start to enter the de-confinement stage of the pandemic.
Phenotype Phebruary: Stay Involved With The Daily Conversations Around Phenotype Development And Evaluations
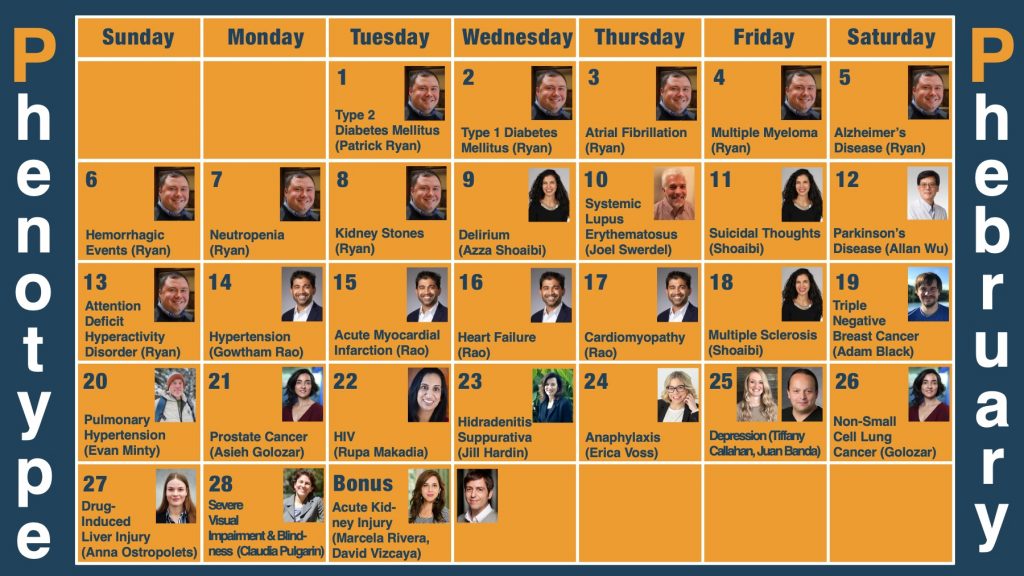 “Phenotype Phebruary” is a community-wide initiative to both develop and evaluate phenotypes for health outcomes that could be investigated by the community. Patrick Ryan introduced this initiative in both a video presentation and a forum post, and each of the conversations around the “28 phenotypes for 28 days” are being held within the OHDSI forums.
“Phenotype Phebruary” is a community-wide initiative to both develop and evaluate phenotypes for health outcomes that could be investigated by the community. Patrick Ryan introduced this initiative in both a video presentation and a forum post, and each of the conversations around the “28 phenotypes for 28 days” are being held within the OHDSI forums.
Our Phenotype Phebruary homepage will provide direct links to each forum post, which is where conversations around each specific phenotype should be held. Please be active in these discussions. There are many ways you can contribute, including joining the conversation, evaluating the cohort definitions in your own data (execute cohort definitions and CohortDiagnostics in your CDM, hare insights you learn from your data on the forums, etc.), or lead your own discussions on phenotypes of interest.
N3C Leadership Presents Work On Extracting OHDSI Concepts from Clinical Narratives for COVID
 Hongfang Liu (Mayo Clinic) and Christopher Chute (Johns Hopkins University) led a session on Extracting OHDSI Concepts from Clinical Narratives for COVID during the Jan. 25 OHDSI Community Call. Following the presentation (approximately 33 minutes), there was a Q&A session.
Hongfang Liu (Mayo Clinic) and Christopher Chute (Johns Hopkins University) led a session on Extracting OHDSI Concepts from Clinical Narratives for COVID during the Jan. 25 OHDSI Community Call. Following the presentation (approximately 33 minutes), there was a Q&A session.
Following an overview of the National COVID Cohort Collaborative (N3C), the presentation focused on Open Health Natural Language Processing and collaborating with the NLP-empowered RECOVER work.
You can watch the full video here, and you can access the slides here.
Vaccine Surveillance Method in Observational Data May Generate High Number Of False Positives
 Worldwide efforts to promote vaccination require reliable evidence about the safety and effectiveness of vaccines to build trust in their use. Regulators and other public health agencies play a critical role in generating and synthesizing evidence across an array of data sources as part of a collective public health infrastructure.
Worldwide efforts to promote vaccination require reliable evidence about the safety and effectiveness of vaccines to build trust in their use. Regulators and other public health agencies play a critical role in generating and synthesizing evidence across an array of data sources as part of a collective public health infrastructure.
One desired component of that system is the use of observational data, such as de-identified electronic health records and administrative claims, to conduct analyses that can identify true adverse events of vaccines as quickly as possible, while simultaneously reducing the chance that analyses generate false positive findings that may stimulate unnecessary worry.
In this context, understanding the reliability of study designs in vaccine surveillance systems is important to ensure that evidence is appropriately used by all stakeholders.
Historical comparator designs, which compare background rates of events in a general population versus observed rates amongst a vaccinated cohort, have been regularly used by regulators and other vaccine safety researchers. Those studies may generate a high number of false positives, according to a recent study published in Frontiers in Pharmacology. The paper, which studied the methods used for surveillance of the H1N1, flu, and other recent vaccines, highlight a need to further evaluate study design in this critical time of COVID-19 vaccine surveillance.
Age-sex adjustment and empirical calibration were among the measures used to produce more reliable surveillance monitoring findings, according to the study “Bias, Precision and Timeliness of Historical (Background) Rate Comparison Methods for Vaccine Safety Monitoring: An Empirical Multi-Database Analysis” led by Xintong Li, a DPhil candidate at the University of Oxford, and supported by the OHDSI and EHDEN open science communities
Our Journey: New Publication Highlighting All Aspects Of OHDSI’s Global Community, Released At #OHDSI2021, Is Available To Global Community
 Our Journey: Where The OHDSI Community Came From, And Where We Are Going, a new publication released at the 2021 OHDSI Global Symposium, is available for download to the global community. This publication highlights all aspects of the global community, ranging from its start and global mission, to its OMOP Common Data Model and global data network, to its collaborative efforts, ranging from study-a-thons and symposia to a full list of more than 360 community publications.
Our Journey: Where The OHDSI Community Came From, And Where We Are Going, a new publication released at the 2021 OHDSI Global Symposium, is available for download to the global community. This publication highlights all aspects of the global community, ranging from its start and global mission, to its OMOP Common Data Model and global data network, to its collaborative efforts, ranging from study-a-thons and symposia to a full list of more than 360 community publications.
The book is divided into 9 chapters (see the graphic to the right), including sections on collaborators and collaborative activities, data standards, methods research, and open-source software. The history of OHDSI publications, including a graphic to show just how collaborative the community is, and our work around COVID-19 is also included, as is a look at future goals for the OHDSI community.
While the download is free, you are able to order the book for yourself, or for your organization. Please reach out to clientservices@abgprint.com for more information.
Ongoing Network Studies, Calls For Collaboration, Highlighted During November Community Call
 Six OHDSI network studies, ranging from those in development to those nearing completion, were presented during the Nov. 16 community call, the second open studies call of 2021. The calls highlighted the breadth of research happening in the community, but also served as calls for collaboration on these important efforts. The individual study presentations are available at the links below.
Six OHDSI network studies, ranging from those in development to those nearing completion, were presented during the Nov. 16 community call, the second open studies call of 2021. The calls highlighted the breadth of research happening in the community, but also served as calls for collaboration on these important efforts. The individual study presentations are available at the links below.
-
-
- Asieh Golozar: Prognostic Significance of Liver Metastasis in Non-Small Cell Lung Cancer
- Leena Elhussein: Redefining Polypharmacy: A Longitudinal Study in Routinely Collected Data
- Noémie Elhadad: Health Equity Research Assessment (HERA) Characterization
- Jacob Zelko: Assessing Health Equity in Mental Healthcare Delivery Using a Federated Network Research Model
- Annika Jodicke and Kristin Kostka: Long COVID Phenotyping and Vaccine Effectiveness Methods
- Erica Voss: Adverse Events of Special Interest within COVID-19 Subjects
-
The LEGEND Initiative — From Its Development And Impact On Hypertension To Future Research Plans Around Type 2 Diabetes
 The LEGEND (Large-scale Evidence Generation and Evaluation across a Network of Databases) Initiative applies high-level analytics to perform observational research on hundreds of millions of patient records within OHDSI’s international database network.
The LEGEND (Large-scale Evidence Generation and Evaluation across a Network of Databases) Initiative applies high-level analytics to perform observational research on hundreds of millions of patient records within OHDSI’s international database network.
LEGEND principles have been applied to studying the effects of treatments for depression, hypertension, and COVID-19, and are being applied to Type 2 diabetes. The clinical impact of LEGEND has already been observed, with important evidence that promotes better health decisions published in Lancet, JAMA Internal Medicine, and Hypertension.
During the Oct. 19 OHDSI Community Call, members of the LEGEND team provided a comprehensive presentation around this initiative, its work around hypertension, and plans around Type 2 diabetes.
Plenaries, Panels, Posters and More! The 2021 OHDSI Global Symposium Home Page Includes All Materials From the Two Main Days
 The 2021 OHDSI Global Symposium featured plenary presentations on both OHDSI’s Impact on the COVID-19 Pandemic, as well as on the Journey to Reliable Evidence. The main days included the State of the Community Presentation, the Collaborator Showcase, and a memorable Closing Ceremony that focused on OHDSI’s work through the perspective of a patient.
The 2021 OHDSI Global Symposium featured plenary presentations on both OHDSI’s Impact on the COVID-19 Pandemic, as well as on the Journey to Reliable Evidence. The main days included the State of the Community Presentation, the Collaborator Showcase, and a memorable Closing Ceremony that focused on OHDSI’s work through the perspective of a patient.
There were also a pair of activities, including the first OHDSI Reproducibility Challenge workshop, and a full-day tutorial on building conceptsets.
All materials from the 2021 Symposium will be shared on this page in the coming weeks. The first two sessions, as well as links to the collaborator showcase presentations, are currently available below.
OHDSI2021 Is On!
 The 2021 OHDSI Symposium began Sunday, Sept. 12, with a full-day tutorial on Building Conceptsets, and then completed its first reproducibility challenge workshop (see image, right). Finally, the main two days began with the first of two plenaries, focusing on OHDSI’s impact on the COVID-19 pandemic. It concludes Wednesday with a plenary focusing on the journey to reliable evidence.
The 2021 OHDSI Symposium began Sunday, Sept. 12, with a full-day tutorial on Building Conceptsets, and then completed its first reproducibility challenge workshop (see image, right). Finally, the main two days began with the first of two plenaries, focusing on OHDSI’s impact on the COVID-19 pandemic. It concludes Wednesday with a plenary focusing on the journey to reliable evidence.
Recordings from all sessions will be posted in the near future, including both reaction panels, which brought together leaders in the field to comment on what they learned from OHDSI presentations. Please continue to check OHDSI.org and our social feeds for an announcement on when those videos will be available.
Daily Agenda For 2021 OHDSI Global Symposium Unveiled, Includes 2 Plenaries, Workshop, Tutorial and Interactive Collaborator Showcase
The daily agenda for the  2021 OHDSI Global Symposium, set for Sept. 12-15, includes a pair of plenaries (Sept. 14: OHDSI Impact on the COVID-19 Pandemic; Sept. 15: Generating Reliable Evidence), the first-ever OHDSI Reproducibility Challenge, a full-day tutorial on Building Conceptsets, and an interactive Collaborator Showcase that will have sessions convenient for collaborators around the world.
2021 OHDSI Global Symposium, set for Sept. 12-15, includes a pair of plenaries (Sept. 14: OHDSI Impact on the COVID-19 Pandemic; Sept. 15: Generating Reliable Evidence), the first-ever OHDSI Reproducibility Challenge, a full-day tutorial on Building Conceptsets, and an interactive Collaborator Showcase that will have sessions convenient for collaborators around the world.
The Symposium, which will be held in the OHDSI MSTeams environment, will begin Sunday, Sept. 12, with the Building Conceptsets tutorial, and then will continue on Monday with the OHDSI Reproducibility Challenge. The traditional symposium will take place over Sept. 14-15, with plenaries and collaborator showcase sessions on both days.
Check out the symposium homepage for more information, and for all registration links.
Community-Led ETL, CDM Sessions Highlight Impact Of Focused Activities Within OHDSI
 Mui Van Zandt and Clair Blacketer, a pair of Titan Award honorees within OHDSI, led a pair of community-driven activities that had multiple benefits during August.
Mui Van Zandt and Clair Blacketer, a pair of Titan Award honorees within OHDSI, led a pair of community-driven activities that had multiple benefits during August.
Van Zandt, a leader in the Asia-Pacific (APAC) community, ran a two-day ETL tutorial August 12-13 that featured seven different sessions (Introduction to ETL & Source Data Analysis, Vocabulary Mapping Part I, Vocabulary Mapping Part II, ETL Specification Writing, ETL Specification Review, Common Issues in ETL Conversion and OMOP ETL Development, and Data Quality Checks). You can watch all of the recordings now on the tutorial homepage.
Blacketer led a two-day CDM Hackathon August 18-19 that welcomed 25 collaborators (see screenshot, right) and focused on our data standards in the OMOP common data model. The team addressed 33 issues, closed 30 pull requests and ended with a fully-functional R package. This effort was devoted to the preparation for OMOP CDM v5.4 and included work on documentation. You can hear about both activities during the Aug. 24 OHDSI Community Call.
HADES Unit-Test-A-Thon Aids Reliability Of Open-Source Tool Packages That Drive Community Research
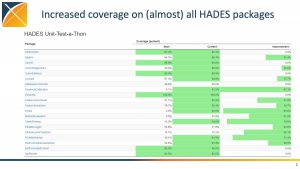 When Martijn Schuemie planned a HADES (Health Analytics Data to Evidence Suite) Unit-Test-A-Thon, his goal was to improve some HADES packages to greater than 80% coverage, and to familiarize more community members with the code and these tools.
When Martijn Schuemie planned a HADES (Health Analytics Data to Evidence Suite) Unit-Test-A-Thon, his goal was to improve some HADES packages to greater than 80% coverage, and to familiarize more community members with the code and these tools.
He didn’t necessarily expect a wide swath of the community to come together for one of OHDSI’s most successful collaborative events in 2021, but he was happy to see such a successful community event. 32 collaborators — ranging from veteran to newcomer — came together to significantly enhance the community-developed tool package that serves as the foundation for almost all community research.
Three collaborators presented about both the event and what they learned from it during a recent community call.
Among Effective Antihypertensive Drugs, Less Popular Choice Is Slightly Safer
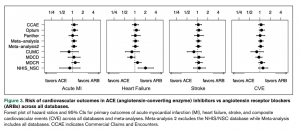 Two types of drugs that are recommended as a first treatment for patients with high blood pressure were found equally effective in improving cardiovascular outcomes, though the more popular type causes slightly more side effects, finds a multinational observational study led by researchers within the Observational Health Data Sciences and Informatics (OHDSI) community.
Two types of drugs that are recommended as a first treatment for patients with high blood pressure were found equally effective in improving cardiovascular outcomes, though the more popular type causes slightly more side effects, finds a multinational observational study led by researchers within the Observational Health Data Sciences and Informatics (OHDSI) community.
The study, which analyzed claims and electronic health data from millions of patients worldwide, is the largest to compare the safety and efficacy of ACE inhibitors and ARBs, two commonly prescribed antihypertensive drugs. It was published online in Hypertension on July 26, 2021.
“Physicians in the United States and Europe overwhelmingly prescribe ACE inhibitors, simply because the drugs have been around longer and tend to be less expensive than ARBs,” says George Hripcsak, MD, Vivian Beaumont Allen Professor and chair of biomedical informatics at Columbia University Vagelos College of Physicians and Surgeons and senior author of the study. Columbia University serves as the coordinating center for the OHDSI community.
“But our study shows that ARBs are associated with fewer side effects than ACE inhibitors. The study was focused on first-time users of these drugs. If you’re just starting drug therapy for hypertension, you might consider trying an ARB first. If you’re already taking an ACE inhibitor and you’re not having any side effects, there is nothing that we found that would indicate a need for a change.”
Design & Preliminary Findings From PROTEUS Study, Generated At #OHDSI2020, Shared With OHDSI Community
 The PROTEUS (Predicting & Recalibrating Outcomes Toward External Understanding Study) study was presented during our July 13 Community Call.
The PROTEUS (Predicting & Recalibrating Outcomes Toward External Understanding Study) study was presented during our July 13 Community Call.
The foundation of this study took place during the 2020 OHDSI Global Symposium. The presentation was led by the two study leaders, David Kent (Professor of Medicine, Clinical & Translational Science Tufts Medical Center) and Benjamin Wessler (Director, Valve Center; Assistant Professor, Tufts University School of Medicine).
Both the video presentation and slides are available for the community.
EUMAEUS Team Presents OHDSI Study On Evaluating Use of Methods for Adverse Event Under Surveillance
 As approved COVID-19 vaccines are rolled out globally, it is likely that safety signals will be identified from spontaneous reports and other data sources. Although some work has been done on the best methods for vaccine safety surveillance, there is a scarcity of information on how these perform in analyses of real-world data. The EUMAEUS (Evaluating Use of Methods for Adverse Event Under Surveillance) team is collaborating to study the comparative performance (bias, precision, and timeliness) of different analytical methods for the study of comparative vaccine safety.
As approved COVID-19 vaccines are rolled out globally, it is likely that safety signals will be identified from spontaneous reports and other data sources. Although some work has been done on the best methods for vaccine safety surveillance, there is a scarcity of information on how these perform in analyses of real-world data. The EUMAEUS (Evaluating Use of Methods for Adverse Event Under Surveillance) team is collaborating to study the comparative performance (bias, precision, and timeliness) of different analytical methods for the study of comparative vaccine safety.
This work was presented during a June community call (video presentation and slides are available). The topics for the presentation are listed below.
-
-
- Literature Review (Lana Lai, Postdoctoral Research Associate, University of Manchester)
- Overview of the EUMAEUS Experiment Design (Marc Suchard, Professor, Department of Biomathematics, UCLA)
- Bias, Precision and Timeliness of Historical Rate Comparison Methods (Xintong Li, DPhil Candidate, University of Oxford)
- Combining Methods in a Safety Surveillance System (Faaizah Arshad, Undergraduate, UCLA)
- Estimating for Two-Dose Vaccines (Ty Stanford, Data Analyst/Bioinformatician, University of South Australia)
- Comparison of Performance Across Methods (Martijn Schuemie, Research Fellow, Epidemiology Analytics, Janssen Research and Development)
-
EHDEN Welcomes 41 New Data Partners Representing 78 Million Patient Records, Brings In Seven New Nations And 12 Focused On Oncology
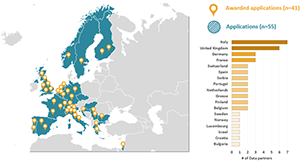 Our colleagues within the EHDEN Consortium recently announced a total of 41 data partners have been selected in our latest open call for data partners. These 41 applications, which came from 55 eligible applications, were selected by the EHDEN Data Source Prioritisation Committee. Combined, the 41 selected data partners represent over 78 million patient records, originating from various care settings.
Our colleagues within the EHDEN Consortium recently announced a total of 41 data partners have been selected in our latest open call for data partners. These 41 applications, which came from 55 eligible applications, were selected by the EHDEN Data Source Prioritisation Committee. Combined, the 41 selected data partners represent over 78 million patient records, originating from various care settings.
This open call had a focus on countries minimally represented in the current EHDEN network, and with this current call, we now have data partners in 7 new countries: Greece, Bulgaria, Israel, Norway, Sweden, Switzerland and Luxembourg. Secondly, the oncology focus was a huge success with 12 new data partners having a focus on oncology while we also welcome several new data partners with a focus on Diabetes Mellitus. EHDEN looks forward to all the new Data Partners joining with the 60 Data Partners across the 16 countries from our prior three calls.
Following the harmonization process, the EHDEN data network will have 98 total data partners across 22 nations.
Largest, Most Extensive Measurement Of Adverse Events Background Rates Can Inform Safety Monitoring Efforts For COVID Vaccines
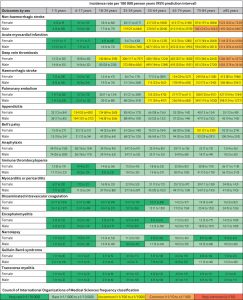 COVID vaccine surveillance efforts are a global priority, but safety monitoring for vaccines should not reflect a single global population. The largest international network study ever completed on the background rates of adverse events of special interest (AESI) being monitored in vaccine surveillance efforts identified that these rates vary substantially by age, sex, and database.
COVID vaccine surveillance efforts are a global priority, but safety monitoring for vaccines should not reflect a single global population. The largest international network study ever completed on the background rates of adverse events of special interest (AESI) being monitored in vaccine surveillance efforts identified that these rates vary substantially by age, sex, and database.
Led by researchers at Oxford University, Columbia University, Erasmus MC, UCLA, and Janssen, an international team of collaborators within the Observational Health Data Sciences and Informatics (OHDSI) network provided a timely reference of the background rates of AESIs in the recent study “Characterising the background incidence rates of adverse events of special interest for covid-19 vaccines in eight countries: multinational network cohort study” published June 14 by The BMJ.
There were significant differences in the observed rates of AESIs based on the age groups and sex of more than 126 million people across four continents and 13 total databases in this observational study. Furthermore, differences were observed across people in distinct databases.
COVID-19 Shows Greater Complications In Youth Than Influenza, Though Fatality Is Rare
 While fatality was fortunately rare, complications including hospitalization, hypoxemia and pneumonia were more frequent in children and adolescents either diagnosed or hospitalized with COVID-19 than with seasonal influenza. Furthermore, labored breathing, loss of smell and gastrointestinal issues were more prevalent symptoms for younger people inflicted with COVID-19 than with influenza.
While fatality was fortunately rare, complications including hospitalization, hypoxemia and pneumonia were more frequent in children and adolescents either diagnosed or hospitalized with COVID-19 than with seasonal influenza. Furthermore, labored breathing, loss of smell and gastrointestinal issues were more prevalent symptoms for younger people inflicted with COVID-19 than with influenza.
These findings, along with that of significant treatment heterogeneity for children/adolescents hospitalized with COVID-19, were presented in the study “30-day outcomes of Children and Adolescents with COVID-19: An International Experience,” published May 28 by Pediatrics.
Early in the pandemic, opinions around the COVID-19 impact on children and adolescents ranged from it being no more than the common flu to fear of its potential impact on lesser-developed immune systems. This OHDSI global network study compared the real-world observational data of more than 242,000 children/adolescents diagnosed and nearly 10,000 hospitalized with COVID-19 to more than 2,000,000 diagnosed with influenza across five countries (France, Germany, South Korea, Spain, United States) to provide a clearer picture of its impact.
PIONEER Prostate Cancer Study-A-Thon Report Provided During May Community Call
 Collaborators from PIONEER, EHDEN and OHDSI came together during a five-day stretch in March to investigate the natural history and outcomes of prostate cancer patients managed with watchful waiting, a conservative management option for prostate cancer patients with a life expectancy of less than 10 years at time of diagnosis. The patient’s disease is ‘watched’ for development of local or systemic progression until they require palliative treatment (care that makes a disease or its symptoms less severe or unpleasant but without removing the cause), with the intention being to maintain quality of life.
Collaborators from PIONEER, EHDEN and OHDSI came together during a five-day stretch in March to investigate the natural history and outcomes of prostate cancer patients managed with watchful waiting, a conservative management option for prostate cancer patients with a life expectancy of less than 10 years at time of diagnosis. The patient’s disease is ‘watched’ for development of local or systemic progression until they require palliative treatment (care that makes a disease or its symptoms less severe or unpleasant but without removing the cause), with the intention being to maintain quality of life.
The aim of this study-a-thon was to assess selection criteria and long-term outcomes of prostate cancer patients on watchful waiting by using an international network of real-world data spanning the years watchful waiting has been a recognized prostate cancer management approach.
Output from that study-a-th0n, as well as ongoing work in this important research topic, was shared in this presentation.
Six Asia-Pacific Regional Chapters Provide Exciting Updates, Share Upcoming Goals, During Recent APAC Call
 Many of the exciting advancements within our global OHDSI community were presented recently at an Asia-Pacific (APAC) community call, when representatives from the six different APAC regional chapters (Australia, China, Japan, Korea, Singapore and Taiwan) provided updates on both their recent accomplishments and upcoming missions.
Many of the exciting advancements within our global OHDSI community were presented recently at an Asia-Pacific (APAC) community call, when representatives from the six different APAC regional chapters (Australia, China, Japan, Korea, Singapore and Taiwan) provided updates on both their recent accomplishments and upcoming missions.
The recording of that call is available here, while a summary can be found within this story.
The APAC community call takes place every other week within our MS Teams environment, and the recordings our posted to our APAC Community homepage. Information for how to join the calls, or any of our regional chapters, are available on that page.
Insufficient Data, Misleading Recommendations Led To Significant Early Heterogeneity In Global COVID-19 Patient Management
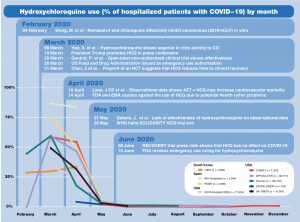 While there was extensive use of drug repurposing throughout the first 10 months of the COVID-19 pandemic, there was substantial heterogeneity over the types of drugs used for treatment purposes globally. Some drugs, including hydroxychloroquine, saw sharp declines in use, while adjunctive therapies grew into a more relied upon method for patient management.
While there was extensive use of drug repurposing throughout the first 10 months of the COVID-19 pandemic, there was substantial heterogeneity over the types of drugs used for treatment purposes globally. Some drugs, including hydroxychloroquine, saw sharp declines in use, while adjunctive therapies grew into a more relied upon method for patient management.
In a number of cases, scientific discovery overturned misconceptions proclaimed via press conferences and social media.
The OHDSI network study “Use of repurposed and adjuvant drugs in hospital patients with covid-19: multinational network cohort study,” published May 11 by The BMJ, provides a global view of drug utilization in routine practice of more than 303,000 hospitalized patients from China, South Korea, Spain and the United States. The study highlights the need for future research on the safety and efficacy of the more commonly used treatments.
Collaborator Spotlight: Evan Minty
 Evan Minty is a general internist and clinical assistant professor at the O’Brien Institute for Public Health at the University of Calgary, Canada. He splits his time between clinical practice, applied clinical informatics with Alberta Health Services (supporting their EMR deployments and decision support development) and research interests in clinical and EMR data science.
Evan Minty is a general internist and clinical assistant professor at the O’Brien Institute for Public Health at the University of Calgary, Canada. He splits his time between clinical practice, applied clinical informatics with Alberta Health Services (supporting their EMR deployments and decision support development) and research interests in clinical and EMR data science.
Evan completed his undergraduate degree in Biophysics at the University of British Columbia, his MSc in Physics at the University of Alberta, his MD and General Internal Medicine residency and fellowship training at the University of Calgary, and his MSc in Biomedical Informatics through Stanford University. He remains onboarded through Stanford University as a research affiliate. He is an active member of the OHDSI community and is in the early stages of developing a Surgery and Perioperative Medicine Workgroup. He discusses that, as well as how he joined the community, how OHDSI impacted his COVID patient management in the last year, and more in this edition of the OHDSI Collaborator Spotlight.
EHDEN Launches Fourth, Largest, Open Call For Data Partners To Map To OMOP
 The EHDEN Consortium announced its fourth open call to all data custodians of electronic health records, claims, hospital and registry data across Europe to join in the goal of standardizing more than 100 million patient records to the OMOP common data model. EHDEN is currently working with 61 data partners following its first three open calls, and it has 26 small-to-medium enterprises (with more expected this year after a recent SME call) trained to work with the data partners in mapping the data to OMOP.
The EHDEN Consortium announced its fourth open call to all data custodians of electronic health records, claims, hospital and registry data across Europe to join in the goal of standardizing more than 100 million patient records to the OMOP common data model. EHDEN is currently working with 61 data partners following its first three open calls, and it has 26 small-to-medium enterprises (with more expected this year after a recent SME call) trained to work with the data partners in mapping the data to OMOP.
Data Partners can benefit from up to a maximum of €100,000 funding towards this mission, and this open call has an overall budget of €5 million. The current open call will run from April 15 to May 13. More information on EHDEN can be found here, and more details on this fourth open call for data partners on the EHDEN website.
The Quiet Driving Force for Observational Research, HADES Empowers a Global Community — Beyond Just OHDSI — To Generate Real-World Evidence
Network Study Updates, Including Pair Of Vaccine-Related Efforts, Presented To Community
 Six OHDSI network studies were presented during an April call to both inform the community about either ongoing or recently completed global research efforts, as well as calling for collaborators to join in these studies.
Six OHDSI network studies were presented during an April call to both inform the community about either ongoing or recently completed global research efforts, as well as calling for collaborators to join in these studies.
These 6-minute breakdowns were recorded and posted to the OHDSI YouTube channel, and all presentations are available below.
Cancer Risk Between H2 Blockers (Seng Chan You)
MSKAI- Musculoskeletal adverse events following hormonal treatment for breast cancer: Cohort Diagnostics to establish feasibility (Jenny Lane)
Covid-19 pandEmic impacts on mental health Related conditions Via multi-database nEtwork: a LongitutinaL Observational (CERVELLO) study (Carmen Olga-Torre)
Alpha-1 blocker for Palliating Inflammatory injury Severity (APIS) study (Aki Nishimura)
Calculating the background rates of adverse events of special interest (AESI) for the COVID vaccines (Xintong Li)
EUMAEUS – Evaluating Use of Methods for Adverse Event Under Surveillance (Martijn Schuemie)
Vaccine Surveillance Efforts Highlighted In Recent OHDSI Community Call
Collaborator Spotlight: Seng Chan You
 Seng Chan You is a medical doctor who majored in internal medicine from Severance Hospital in Seoul, South Korea. He received his Master of Medical Science at the same university, and then he earned his PhD in the Department of Biomedical Informatics at Ajou University, and he has been a leader in the OHDSI community over the last several years. Honored with the 2018 OHDSI Titan Award for Clinical Application, Chan has led the expansion of the OHDSI network into the Asia-Pacific (APAC) region, including hosting the 2019 OHDSI Korea Symposium and assisting in the development of the 2020 OHDSI APAC Symposium.
Seng Chan You is a medical doctor who majored in internal medicine from Severance Hospital in Seoul, South Korea. He received his Master of Medical Science at the same university, and then he earned his PhD in the Department of Biomedical Informatics at Ajou University, and he has been a leader in the OHDSI community over the last several years. Honored with the 2018 OHDSI Titan Award for Clinical Application, Chan has led the expansion of the OHDSI network into the Asia-Pacific (APAC) region, including hosting the 2019 OHDSI Korea Symposium and assisting in the development of the 2020 OHDSI APAC Symposium.
Chan, a leader in several OHDSI workgroups, recently led the effort to have South Korean HIRA data available to the OHDSI global community during the COVID-19 study-a-thon. He c0-led the study “Association of Ticagrelor vs Clopidogrel With Net Adverse Clinical Events in Patients With Acute Coronary Syndrome Undergoing Percutaneous Coronary Intervention” which was published in JAMA in October 2020, and has co-authored many OHDSI network studies, including the LEGEND Hypertension study published in The Lancet.
Chan recently accepted a new position as a Research Assistant Professor at his alma mater. He recently shared thoughts on this new role, OHDSI’s growth in the Asia-Pacific region, leading the COVID-19 data sharing efforts, and plenty more in the latest edition of the OHDSI Collaborator Spotlight.
HL7 International and OHDSI Announce Collaboration to Provide Single Common Data Model for Sharing Information in Clinical Care and Observational Research
 Health Level Seven International (HL7®) and the Observational Health Data Sciences and Informatics (OHDSI) network today announced a collaboration to address the sharing and tracking of data in the healthcare and research industries by creating a single common data model. The organizations will integrate HL7 Fast Healthcare Interoperability Resources (FHIR®) and OHDSI’s Observational Medical Outcomes Partnership (OMOP) common data model to achieve this goal.
Health Level Seven International (HL7®) and the Observational Health Data Sciences and Informatics (OHDSI) network today announced a collaboration to address the sharing and tracking of data in the healthcare and research industries by creating a single common data model. The organizations will integrate HL7 Fast Healthcare Interoperability Resources (FHIR®) and OHDSI’s Observational Medical Outcomes Partnership (OMOP) common data model to achieve this goal.
The organizations will align their standards to capture data in a clearly defined way into a single common data model. This will allow clinicians as well as researchers to pull data from multiple sources and compile it in the same structure without degradation of the information. This endeavor has global implications with the potential to permit the clinical community to define the elements they need, package and share them in a consistent single structure.
“We are excited to have the OHDSI community join this partnership with HL7 to evolve community standards around observational research and clinical care,” said George Hripcsak, MD, MS, OHDSI’s coordinating center director. “These standards set the foundation for our mission of global, open-science research, and this partnership will accelerate the development of effective and safe treatments for diseases facing today’s global population.
HL7 International CEO Dr. Charles Jaffe, M.D., Ph.D., underscored the significance of this partnership. “The Covid-19 pandemic has emphasized the need to share global health and research data.” He continued, “Collaboration with OHDSI is critical to solving this challenge and will help our mutual vision of a world in which everyone can securely access and use the right data when and where they need it.”
CHARYBDIS 2.0, Early SCYLLA Findings, Vaccine AESI Study Framework Discussed In OHDSI COVID-19 Research Presentation
 OHDSI research on COVID-19, which began with a memorable global study-a-thon last March, continues to progress in various ways throughout the world. Leaders in three of the largest network studies on the disease joined the Feb. 23 OHDSI community call to provide updates on these projects.
OHDSI research on COVID-19, which began with a memorable global study-a-thon last March, continues to progress in various ways throughout the world. Leaders in three of the largest network studies on the disease joined the Feb. 23 OHDSI community call to provide updates on these projects.
Kristin Kostka (IQVIA), Dani Prieto-Alhambra (University of Oxford) and George Hripcsak (Columbia University) gave presentations on ongoing work in both Project CHARYBDIS and SCYLLA, as well as OHDSI’s work with the FDA on the safety and effectiveness surveillance of the COVID-19 vaccines.
Videos and slides from each of the presentations are available here or on the Community Calls page.
World’s Largest Study on ACE Inhibitors, ARBs Shows No Increased Patient Risk of COVID-19 Diagnosis, Complications
EHDEN Network Expands to 62 Data Partners Across 16 Nations Following Latest Open Call
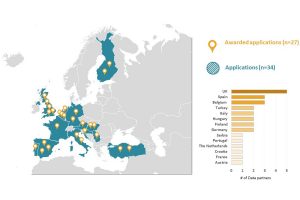 A critical aspect of the EHDEN project is for it to collaborate with data partners, such as hospitals, primary care providers, networks of both, regional datasets, claims databases and cohorts, with a view to harmonizing their clinical data locally to a common data model. Key to the success of the collaboration is the support of federated research via standardized analytical tools.
A critical aspect of the EHDEN project is for it to collaborate with data partners, such as hospitals, primary care providers, networks of both, regional datasets, claims databases and cohorts, with a view to harmonizing their clinical data locally to a common data model. Key to the success of the collaboration is the support of federated research via standardized analytical tools.
EHDEN, within the Innovative Medicines Initiative (IMI), is a public-private partnership responding to the need to improve our speed to answers in real-world research. To this end, it has been creating a federated network, in collaboration with Data Partners, Small-to-Medium Size Enterprises (SMEs), the OHDSI framework and network, researchers, regulatory authorities and many others.
The EHDEN project is very pleased to announce the following 27 new Data Partners have been selected in our third open call for data partners. Collectively, our Data Partner Network now has 62 Data Partners, covering sixteen countries and have greater than 200 million anonymized patient health records that are being mapped to the common data model.
OHDSI Awarded $10 Million FDA Contract to Support Safety/Effectiveness Surveillance of Vaccines, Other Biological Products
 Researchers within the Observational Health Data Services and Informatics (OHDSI) community were recently awarded a $10 million contract from the U.S. Food and Drug Administration (FDA) to provide support to the Biologics Effectiveness and Safety (BEST) program, which was launched by the FDA Center for Biologics Evaluation and Research (CBER) in 2017.
Researchers within the Observational Health Data Services and Informatics (OHDSI) community were recently awarded a $10 million contract from the U.S. Food and Drug Administration (FDA) to provide support to the Biologics Effectiveness and Safety (BEST) program, which was launched by the FDA Center for Biologics Evaluation and Research (CBER) in 2017.
The lead research team, primarily comprised of OHDSI personnel from Columbia University, UCLA, and Northeastern University, will provide support to the BEST system in its mission to conduct safety and effectiveness surveillance of biologic products (vaccines, blood and blood products, tissues and advanced therapeutics).
Specific means of FDA support through this grant will include serving in a convening role to 1) develop methods related to using observational data from electronic health records and administrative claims to study the effectiveness and safety of biologics, 2) work collaboratively with FDA staff to plan, develop, coordinate, host and convene meetings and workshops, and 3) educate FDA staff and external stakeholders on the BEST infrastructure, capabilities, and applications that serve FDA and stakeholder needs.
Preferred ACS Treatment Not Associated With Better Patient Outcomes In Contemporary Clinical Practice According To Recent Multi-National Observational Study
 Ticagrelor, when compared with clopidogrel, was not associated with better outcomes for patients with acute coronary syndrome (ACS) treated with percutaneous coronary intervention in contemporary routine clinical practice according to a study published recently by the Observational Health Data Sciences and Informatics (OHDSI) community.
Ticagrelor, when compared with clopidogrel, was not associated with better outcomes for patients with acute coronary syndrome (ACS) treated with percutaneous coronary intervention in contemporary routine clinical practice according to a study published recently by the Observational Health Data Sciences and Informatics (OHDSI) community.
These findings raise doubts about current clinical guidelines for ACS treatment, which recommend ticagrelor over clopidogrel based on the findings of a single large sponsor-initiated clinical trial, PLATO, which was published in 2009.
This global network study, which included more than 60,000 patients with acute coronary syndrome who underwent PCI from two United States electronic health record-based databases and one nationwide South Korean database, is intended to provide greater detail about the characteristics of patients suffering from the disease, and also to help inform decision-making around the care of patients with ACS.
The study “Association of Ticagrelor vs Clopidogrel With Net Adverse Clinical Events in Patients With Acute Coronary Syndrome Undergoing Percutaneous Coronary Intervention” was published Oct. 27, 2020 by JAMA.
OHDSI2020 Global Symposium Highlights Cutting-Edge Research, Community Advances; All Presentations, Collaborator Showcase Research Is Now Available
The 2020 OHDSI Global Symposium brought together a global research community for 18 hours of open science, international collaboration and community fun on Oct. 19. The day included presentations from community members, panels that brought together leaders from a variety of major healthcare organizations, as well as network sessions, the annual collaborator showcase, and plenty more.
Please check out this page for complete updates from the day, and follow the OHDSI Twitter and LinkedIn feeds for the #OHDSISocialShowcase, which will highlight all presentations from the 2020 Collaborator Showcase.
Hospitalized COVID-19 Patients Shown To Be Younger, Healthier Than Influenza Patients Per Recent Global Observational Health Study
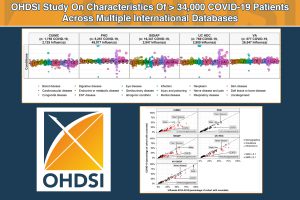 Patients hospitalized with COVID-19 were more likely male, younger, and, in both the US and Spain, had fewer comorbidities and lower medication use than hospitalized influenza patients according to a recent study published by the Observational Health Data Sciences and Informatics (OHDSI) community.
Patients hospitalized with COVID-19 were more likely male, younger, and, in both the US and Spain, had fewer comorbidities and lower medication use than hospitalized influenza patients according to a recent study published by the Observational Health Data Sciences and Informatics (OHDSI) community.
This global network study, which included more than 34,000 COVID-19 patients from across three continents, is intended to provide greater detail about the characteristics of patients suffering from the disease, and also to help inform decision-making around the care of hospitalized patients.
The study “Deep phenotyping of 34,128 adult patients hospitalized with COVID-19 in an international network study” was published Oct. 6 by Nature Communications and is available here.
Patients hospitalized with COVID-19 were more typically male in the US and Spain, but more often female in South Korea. The ages of patients varied, but in Spain and the US, the most common age groups were between 60 to 75. Patients hospitalized with influenza were typically older than those hospitalized with COVID-19, and more likely to be female.
OHDSI Symposium Panel – Building Trust: Evidence and its Communication
 Leaders from around the healthcare community will share their insights during a panel discussion entitled “Building Trust: Evidence and its Communication” during a highlight event of the 2020 OHDSI Global Symposium, which will be held Oct. 19, 2020. The panel will be held at 1 pm ET during the symposium, which is free for all attendees.
Leaders from around the healthcare community will share their insights during a panel discussion entitled “Building Trust: Evidence and its Communication” during a highlight event of the 2020 OHDSI Global Symposium, which will be held Oct. 19, 2020. The panel will be held at 1 pm ET during the symposium, which is free for all attendees.
This panel is scheduled to include:
• Amy Abernethy, MD, PhD, the Principal Deputy Commissioner of the U.S. FDA
• Patti Brennan, RN, PhD, the Director of the National Library of Medicine, NIH
• Magdalena Skipper, PhD, Editor in Chief, Nature
• Deborah Nelson, JD, Associate Professor of Investigative Journalism, University of Maryland
• Roni Caryn Rabin, MS, science reporter for the New York Times
George Hripcsak, MD, MS, Professor of Biomedical Informatics at Columbia University, will serve as moderator for this panel, which will take place at 1 pm ET during the main symposium
OHDSI Announces 18-Hour Global Symposium Schedule
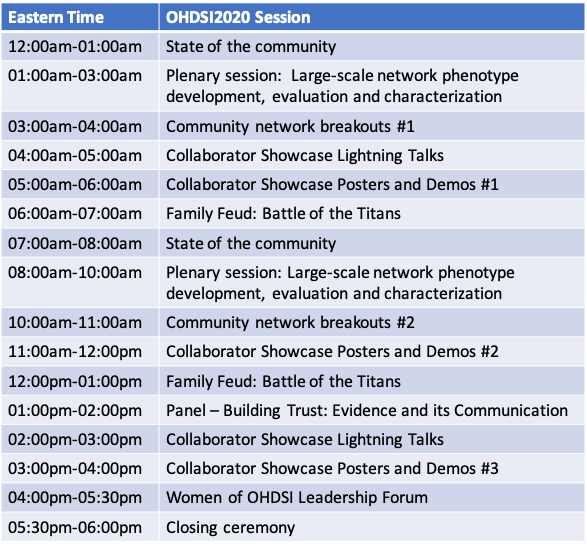 The OHDSI community will welcome both network veterans and newcomers from all parts of the world to join in an 18-hour celebration of open science, international collaboration, and community fun on Monday, Oct. 19 during the 2020 OHDSI Global Symposium.
The OHDSI community will welcome both network veterans and newcomers from all parts of the world to join in an 18-hour celebration of open science, international collaboration, and community fun on Monday, Oct. 19 during the 2020 OHDSI Global Symposium.
Part of a four-day event that will include both a day of tutorials on Oct. 18 and a two-day study-a-thon on Oct. 20-21, the global symposium will include many of the events that OHDSI veterans look forward to each year at the annual symposium … and a few surprises as well.
The symposium will be held virtually over Microsoft Teams, and registration is available here. Once again, there is no charge to attending the OHDSI Symposium.
Starting at midnight ET, our international community will have the opportunity to experience the full excitement of our 2020 symposium. Our Asia-Pacific collaborators will start in the afternoon, while the early risers in Europe can get a jump on their day with the state of the community address. As the symposium continues through the day, our European friends can enjoy panel discussions, poster sessions and a game show in the evening as our North American collaborators are in the middle of their symposium journey!
Collaborator Spotlight: Mui Van Zandt
Mui Van Zandt is a Director of Product Development at IQVIA, and she manages the OMOP Factory. Mui’s areas of expertise include software development, data conversions, agile process, and project management. Mui has gained extensive knowledge working on large patient databases in the OMOP model and the standard vocabularies that are needed to support these conversions.
Mui is an active contributor to the community through various OHDSI working groups. She is one of the co-leaders of the China OMOP CDM/Vocabulary working group. She leads two of the sub-working groups within the THEMIS working group. She has and continues to perform OMOP tutorial training to many different organizations and conferences, such as the OHDSI Symposiums, the China Hackathons, and individual universities.
A veteran of the OHDSI community, Mui recently shared some thoughts on her journey with the community, her work on the CDM and vocabularies, OHDSI progress, and more during the latest edition of the Collaborator Spotlight.
Podcast: Jenny Lane on the OHDSI Hydroxychloroquine Study
 Jenny Lane, co-lead author of the recently published “Risk of hydroxychloroquine alone and in combination with azithromycin in the treatment of rheumatoid arthritis: a multinational, retrospective study,” discussed the study and the 2020 journey of hydroxychloroquine during the debut episode of the OHDSI podcast, which is available below, as well as on Apple Podcasts, Podbean and other podcast apps.
Jenny Lane, co-lead author of the recently published “Risk of hydroxychloroquine alone and in combination with azithromycin in the treatment of rheumatoid arthritis: a multinational, retrospective study,” discussed the study and the 2020 journey of hydroxychloroquine during the debut episode of the OHDSI podcast, which is available below, as well as on Apple Podcasts, Podbean and other podcast apps.
Lane opens with a discussion on everything that went into the study, which was generated during the OHDSI COVID-19 study-a-thon in March, but she also talks about her own personal connection to hydroxychloroquine and its connection with rheumatoid arthritis, as well as the early clinical and methodological impact of the study during its preprint stage. She also discusses how open science and collaboration impacted this global study, and she provided insight on a recent OHDSI preprint she led, a study on the risk of depression, suicide and other psychological impacts in hydroxychloroquine treatment.
You can read more about the study here.
You can listen to the podcast below. Following a brief introduction, Jenny Lane joins the podcast at the 1:55 mark.
OHDSI Collaborators Publish 10 Principles Of LEGEND Project
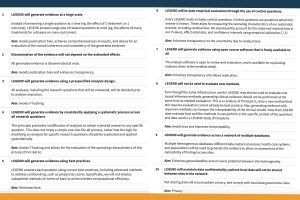 Evidence derived from existing health-care data, such as administrative claims and electronic health records, can fill evidence gaps in medicine. However, many claim such data cannot be used to estimate causal treatment effects because of the potential for observational study bias; for example, due to residual confounding. Other concerns include P hacking and publication bias.
Evidence derived from existing health-care data, such as administrative claims and electronic health records, can fill evidence gaps in medicine. However, many claim such data cannot be used to estimate causal treatment effects because of the potential for observational study bias; for example, due to residual confounding. Other concerns include P hacking and publication bias.
In response, an international group of OHDSI collaborators launched the Large-scale Evidence Generation and Evaluation across a Network of Databases (LEGEND) research initiative. Its mission is to generate evidence on the effects of medical interventions using observational health-care databases while addressing the aforementioned concerns by following a recently proposed paradigm. We define 10 principles of LEGEND that enshrine this new paradigm, prescribing the generation and dissemination of evidence on many research questions at once; for example, comparing all treatments for a disease for many outcomes, thus preventing publication bias. These questions are answered using a prespecified and systematic approach, avoiding P hacking. Best-practice statistical methods address measured confounding, and control questions (research questions where the answer is known) quantify potential residual bias. Finally, the evidence is generated in a network of databases to assess consistency by sharing open-source analytics code to enhance transparency and reproducibility, but without sharing patient-level information.
Here we detail the LEGEND principles and provide a generic overview of a LEGEND study. Our companion paper highlights an example study on the effects of hypertension treatments, and evaluates the internal and external validity of the evidence we generate.
Largest Global Study on Hydroxychloroquine Safety Finds Increased Cardiovascular Risk with Azithromycin
The combination of hydroxychloroquine (HCQ) and azithromycin (AZM) has been linked to significant cardiovascular risks, including mortality, in the largest safety study ever performed on both HCQ and HCQ+AZM. This network study, led by the Observational Health Data Sciences and Informatics community, was recently published in Lancet Rheumatology.
In patients with rheumatoid arthritis, HCQ treatment in the short term (30 days) was found to not carry an excess risk of complications associated with its use, but HCQ treatment in the long term had a 65% relative increase in cardiovascular-related mortality, compared to sulfasalazine.
HCQ + AZM had a cardiovascular mortality risk that was more than twice (2.19) as high as the comparative treatment even in the short term based on findings from more than 320,000 users of that combination therapy. This treatment also produced a 15-20% increased rate of angina/chest pain and heart failure.
The full paper is available here.
OHDSI Obtains Grant Towards Global Research On COVID-19 Treatments
 An international cohort of OHDSI collaborators obtained a grant from the COVID-19 Therapeutics Accelerator to lead an effort to compare the effectiveness of treatments, including corticosteroids such as dexamethasone, under current evaluation for COVID-19 across an international observational data network. The Therapeutics Accelerator is an initiative launched by the Bill & Melinda Gates Foundation, Wellcome, and Mastercard to speed up the response to the COVID-19 pandemic by identifying, assessing, developing, and scaling up treatments.
An international cohort of OHDSI collaborators obtained a grant from the COVID-19 Therapeutics Accelerator to lead an effort to compare the effectiveness of treatments, including corticosteroids such as dexamethasone, under current evaluation for COVID-19 across an international observational data network. The Therapeutics Accelerator is an initiative launched by the Bill & Melinda Gates Foundation, Wellcome, and Mastercard to speed up the response to the COVID-19 pandemic by identifying, assessing, developing, and scaling up treatments.
Researchers from the University of Oxford, Columbia University, UCLA and Erasmus University Medical Center are leading this work through Project SCYLLA (SARS-Cov-2 Large-scale Longitudinal Analyses), one of the emerging efforts to come from OHDSI’s global work surrounding COVID-19 research.
“We are so appreciative that the donors of the Therapeutics Accelerator support our work around studying the effectiveness and safety of potential COVID-19 medicines,” says Daniel Prieto-Alhambra, Professor of Pharmaco- and Device Epidemiology at Oxford and co-PI on the project. “Their funding will help us learn which treatments are showing potential throughout an international cohort of patients. Every ounce of knowledge is a building block that will eventually lead us out of this global crisis.”
Collaborator Spotlight: Anthony Sena
 Anthony Sena is an Associate Director of Epidemiology Analytics at Janssen Research and Development where he architects software solutions and data platforms for the analysis and application of observational data sources. Anthony’s areas of expertise include web application development, data modeling, information visualization, technology infrastructure, project management, and informatics. A collaborator on a number of open-source software solutions in OHDSI and one of the co-leads of the ATLAS & WebAPI working group, he has taken a prominent role in the recent CHARYBDIS Project, a characterization study to understand the disease natural history of COVID-19. His focus is on expanding the capabilities of the OHDSI open-source solution architecture to enable transparent and reproducible research using observational data.
Anthony Sena is an Associate Director of Epidemiology Analytics at Janssen Research and Development where he architects software solutions and data platforms for the analysis and application of observational data sources. Anthony’s areas of expertise include web application development, data modeling, information visualization, technology infrastructure, project management, and informatics. A collaborator on a number of open-source software solutions in OHDSI and one of the co-leads of the ATLAS & WebAPI working group, he has taken a prominent role in the recent CHARYBDIS Project, a characterization study to understand the disease natural history of COVID-19. His focus is on expanding the capabilities of the OHDSI open-source solution architecture to enable transparent and reproducible research using observational data.
Prior to joining Janssen Research and Development, Anthony held many leadership and technical roles of increasing responsibility across a range of business sectors including energy, pharmaceuticals, retail and financial services. He recently discussed his journey to OHDSI, his work with open-source tools, and some of his most important projects, during the latest Collaborator Spotlight.
EMA Announces Partnership With EHDEN To Monitor COVID-19 Treatments
 The European Medicines Agency recently announced an infrastructure to support the monitoring of the efficacy and safety of COVID-19 treatments and vaccines when used in day-to-day clinical practice. The EMA will work with EHDEN on this initiative to establish a European framework and research network for the conduct of multicenter cohort studies on the use of medicines in COVID-19 patients.
The European Medicines Agency recently announced an infrastructure to support the monitoring of the efficacy and safety of COVID-19 treatments and vaccines when used in day-to-day clinical practice. The EMA will work with EHDEN on this initiative to establish a European framework and research network for the conduct of multicenter cohort studies on the use of medicines in COVID-19 patients.
This will be a one-year EMA-funded project, which includes data sources from eight European countries standardized to the OMOP-Common Data Model, and is contracted to IQVIA as the coordinating partner. OHDSI and EHDEN leaders from both the Erasmus Medical Center and the University of Oxford will help drive this important work.
“This is precisely what EHDEN was set up for,” says Peter Rijnbeek, associate professor at Erasmus. “We are creating an international, open science network in Europe, based on a common data model, standardized analytics, tools, and methodologies. It’s exciting to participate in this project and further strengthen our collaboration with the EMA.”
EMA References OHDSI Efforts In Latest Revision Of Scientific Best Practices In Observational Research
 The European Medicines Agency (EMA) provided guidance for scientific best practices in observational research in the recently released 8th revision of its guidelines. This work aligns with OHDSI’s mission, and our global community was proud to see multiple OHDSI efforts, including a pair of COVID-19 studies, have informed and supported their recommendations.
The European Medicines Agency (EMA) provided guidance for scientific best practices in observational research in the recently released 8th revision of its guidelines. This work aligns with OHDSI’s mission, and our global community was proud to see multiple OHDSI efforts, including a pair of COVID-19 studies, have informed and supported their recommendations.
The foreword in “The European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP) Guide on Methodological Standards in Pharmacoepidemiology” highlighted a pair of community preprints currently undergoing peer review.
2020 EU Symposium Collaborators Share Research As Part Of Virtual Showcase
 Though the 2020 European Symposium was canceled and replaced with the COVID-19 Study-A-Thon, many community members still shared their research that had been accepted for the Collaborators Showcase. Those posters, along with abstracts and other links, were highlighted over the OHDSI social platforms, and are all available here.
Though the 2020 European Symposium was canceled and replaced with the COVID-19 Study-A-Thon, many community members still shared their research that had been accepted for the Collaborators Showcase. Those posters, along with abstracts and other links, were highlighted over the OHDSI social platforms, and are all available here.
This research comes from around the world and highlights the breadth and variety of OHDSI research in a pre-COVID world.
No Clear Risk For COVID-19 Diagnosis or Hospitalization From ACE/ARB Use According To Recent OHDSI Preprint
 The Observational Health Data Sciences and Informatics (OHDSI) collaboration released a preprint entitled “Renin-angiotensin system blockers and susceptibility to COVID-19: a multinational open science cohort study.”
The Observational Health Data Sciences and Informatics (OHDSI) collaboration released a preprint entitled “Renin-angiotensin system blockers and susceptibility to COVID-19: a multinational open science cohort study.”
Preprints are preliminary reports of work that have not been certified by peer review. They should not be relied on to guide clinical practice or health-related behavior and should not be reported in news media as established information.
The preprint is available here.
There was no clear increased risk of COVID-19 diagnosis, hospitalization, or subsequent complications found for users of either angiotensin-converting enzyme inhibitors (ACEs) or angiotensin receptor blockers (ARBs) among a multinational cohort of more than 1.1 million patients using antihypertensives.
This study, the most comprehensive one to date of COVID-19 susceptibility risks for antihypertensive users, examined electronic health records from a trio of data sources from the United States and Spain (Columbia University Irving Medical Center, the Department of Veteran Affairs and SIDIAP) to conduct a systematic cohort study of ACE, ARB, calcium channel blocker (CCB) and thiazide diuretic (THZ) users.
“We currently lack reliable, transparent and generalizable evidence to inform antihypertensive choice in light of COVID-19,” says Dr. Marc Suchard, a professor at UCLA and research team leader. “This work strives to openly and reproducibly leverage real-world data to help.”
As a consequence, the study, powered by open-source tools and global collaboration within the OHDSI community, reinforces current clinical guidelines surrounding antihypertensive therapy. The findings indicate that patients should continue their ACE or ARB therapy, despite early concerns about potential risks.
Furthermore, the findings showed no clinical reason to switch from an ARB to ACE to minimize COVID-19 risk. “Based on our results, if there is a risk difference, it’s marginal and would be very challenging to further refine outside such a large-scale international study,” Dr. Suchard says.
The International COVID-ACE Receptor Inhibition Utilization and Safety (ICARIUS) protocol, code, and results are all available for further exploration at https://github.com/ohdsi-studies/Covid19Icarius.
Collaborator Spotlight: Kees van Bochove
 Kees van Bochove is the founder and CEO of The Hyve, a 40-person international company dedicated to the support and facilitation of open source, open standards, and open data in biomedical research. He studied Computer Science at the University of Utrecht and Bioinformatics at VU University Amsterdam and Tufts University in Boston. Kees is active in many open-source software development communities such as i2b2/tranSMART, cBioPortal, OHDSI, RADAR, Galaxy, etc. Through his many years of experience in open source software and standards development in biomedical informatics, Kees has gained a deep understanding of all aspects of collaborative open source development and open data science, including open source community building and governance, software quality, and sustainability requirements, data workflows, etc.
Kees van Bochove is the founder and CEO of The Hyve, a 40-person international company dedicated to the support and facilitation of open source, open standards, and open data in biomedical research. He studied Computer Science at the University of Utrecht and Bioinformatics at VU University Amsterdam and Tufts University in Boston. Kees is active in many open-source software development communities such as i2b2/tranSMART, cBioPortal, OHDSI, RADAR, Galaxy, etc. Through his many years of experience in open source software and standards development in biomedical informatics, Kees has gained a deep understanding of all aspects of collaborative open source development and open data science, including open source community building and governance, software quality, and sustainability requirements, data workflows, etc.
Kees has been involved in OHDSI initially via the IMI EMIF project starting in 2013 and has been building a team around OMOP/OHDSI through the EMIF collaboration, working with a.o. Janssen and ErasmusMC as well as by providing OMOP mapping and OHDSI installation and support services to several pharmaceutical companies. Kees was also involved in planning the first OHDSI Europe meeting in March 2018, and he hosted a subsequent follow-up workshop to introduce OHDSI to a number of European national health-data initiatives (from a.o. The Netherlands, Germany, Switzerland, and Denmark) in May 2018. The Hyve is also leading WP4 in the IMI EHDEN project, which is one of the largest components of this important project, laying the technical groundwork and building data conversion and quality management tools for further developing the European and global OHDSI community.
(Click the link in the headline for a Q&A with Kees)
OHDSI Presents Study-A-Thon Findings At 2020 EULAR E-Congress
 OHDSI collaborators shared important findings generated from the 2020 Barcelona Study-A-Thon at the recent 2020 EULAR E-Congress, held June 3-6, 2020.
OHDSI collaborators shared important findings generated from the 2020 Barcelona Study-A-Thon at the recent 2020 EULAR E-Congress, held June 3-6, 2020.
“It was an honor for our team to share our findings at the 2020 EULAR Congress,” said Daniel Prieto-Alhambra, who led the Barcelona Study-A-Thon, and who also served on the EULAR Congress Programme Committee. “The experience in Barcelona was an enjoyable event, but the real-world studies and findings generated from that week can have a powerful impact on the field of rheumatology, and we couldn’t be prouder of that.”
Posters, slides and Cynthia Yang’s oral presentation can all be found by clicking here.
Seek COVER, First COVID-19 Prediction Study Generated During OHDSI Study-A-Thon, Released in OHDSI Preprint
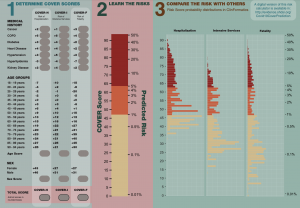 The first Patient-Level Prediction paper has been sent to MedRxiv and is being submitted for peer review. This study “Seek COVER: Development and validation of a personalized risk calculator for COVID-19 outcomes in an international network” is designed to inform individual behavioral choices and help design shielding strategies during de-confinement.
The first Patient-Level Prediction paper has been sent to MedRxiv and is being submitted for peer review. This study “Seek COVER: Development and validation of a personalized risk calculator for COVID-19 outcomes in an international network” is designed to inform individual behavioral choices and help design shielding strategies during de-confinement.
Led by co-first authors Ross Williams and Aniek Markus, the team designed a nine-predictor COVID-19 Estimated Risk (COVER) model that was validated using more than 43,000 COVID patients (following initial development and validation using more than 6.8 million patients with influenza or flu-like symptoms). This model predicts hospitalization, intensive services, and death, and can help provide reassurance for low-risk patients, while shielding high-risk patients, as many start to enter the de-confinement stage of the pandemic.
Peter Rijnbeek is the corresponding author, and both he and Jenna Reps are co-last authors. Overall there are 43 authors involved in the study, once again highlighting the global collaborative nature of the OHDSI community. Congratulations to all who were involved in this work.
Collaborator Spotlight: Kristin Kostka
 Kristin Kostka is an Associate Director at IQVIA running the OMOP Data Network and a perennial collaborator in the OHDSI community. In her work, Kristin partners with hospitals, payers and healthcare providers to help organizations unlock the power of institutional data and connect with the world’s largest observational health data network.
Kristin Kostka is an Associate Director at IQVIA running the OMOP Data Network and a perennial collaborator in the OHDSI community. In her work, Kristin partners with hospitals, payers and healthcare providers to help organizations unlock the power of institutional data and connect with the world’s largest observational health data network.
Kristin has over 10 years of experience leading real-world evidence generation studies, designing and implementing enterprise patient data lakes, conducting large-scale multinational clinical trials and preparing regularly submissions. Within OHDSI, Kristin sits on the OHDSI Steering Committee, the US Symposium Scientific Committee, the Women of OHDSI group, the OHDSI Study Nurture Committee and regularly leads OHDSI network studies. Kristin co-authored three chapters of the Book of OHDSI (Where to Begin, Defining Cohorts and OHDSI Network Research). Her OHDSI passion project is the idea of “studies on studies” — evaluating the best way to disseminate evidence once its generated. Kristin currently serves as a member of the OHDSI COVID-19 Study-a-thon Core Team facilitating follow-on work from the recent virtual study-a-thon. She is also a Co-Principal Investigator on Project CHARYBDIS (Characterizing Health Associated Risks, and Your Baseline Disease In SARS-COV-2).
Kristin is a recipient of many industry awards, including the 2020 Elon University Young Alumni Council “Top 10 Under 10” Alumni Award, a 2018 OHDSI Titan Award for Community Collaboration, a 3-time recipient of Deloitte Outstanding Performance Award and an 8-time recipient of the Deloitte Applause Award for exceptional client service. She holds a Bachelor’s degree in Exercise Science from Elon University and a Master’s in Public Health in Epidemiology from Boston University School of Public Health.
(Click the link in the headline for a Q&A with Kristin)
Multi-Institutional Characterization Study on Hospitalized COVID Patients, And Comparison To Hospitalized Influenza Patients, Released in OHDSI Preprint
 The Observational Health Data Sciences and Informatics (OHDSI) collaboration released a preprint on an international characterization of patients hospitalized with COVID-19 and a comparison with those previously hospitalized with influenza.
The Observational Health Data Sciences and Informatics (OHDSI) collaboration released a preprint on an international characterization of patients hospitalized with COVID-19 and a comparison with those previously hospitalized with influenza.
Preprints are preliminary reports of work that have not been certified by peer review. They should not be relied on to guide clinical practice or health-related behavior and should not be reported in news media as established information.
The preprint is available here.
The characteristics (demographics, prior conditions and medication use) of more than 6,800 COVID-positive patients from four databases (Columbia, Stanford, the Department of Veterans Affairs and the South Korean Health Insurance Review & Assessment) were reported in this study and were compared to more than 52,000 patients hospitalized with influenza between 2014-19.
Compared to 52,422 individuals hospitalized with influenza, patients admitted with COVID-19 were more likely male, younger, and, in the US, had fewer comorbidities and lower medication use.
EHDEN Academy Goes Live as Free Resource in Real-World Health Research
 The European Health Data & Evidence Network (EHDEN) today announced the launch of the EHDEN Academy in Europe as an online educational resource for anyone working in the domain of real-world data use, and real-world evidence generation, with the aim of becoming a primary resource.
The European Health Data & Evidence Network (EHDEN) today announced the launch of the EHDEN Academy in Europe as an online educational resource for anyone working in the domain of real-world data use, and real-world evidence generation, with the aim of becoming a primary resource.An IMI2 (Innovative Medicines Initiative) EHDEN project, the EHDEN Academy’s goal is to build upon the foundations of the EHDEN project and its collaboration with the OHDSI community. The EHDEN Academy aims to be a resource for all those who generate and utilize data, work technically with it (e.g. ETL and mapping of data to the OMOP common data model), and are involved in the methodological development and use of standardized analytical tools within the OHDSI framework.
As the current SARS-CoV-2 pandemic is showing us, the ability to collaborate and work with real-world data is critical to clinical decision-making, planning, and management decisions. As such, EHDEN is responding to the pandemic via its involvement in the OHDSI COVID-19 Virtual Study-a-thon, a COVID-19 Data Partner call, also supporting education in using real-world evidence at this critical time via the EHDEN Academy.
88 Hours: OHDSI’s Signature Moment
 The time was meant for highlighting OHDSI capabilities, not testing them. The hours were meant to sharing global research, not sharing in global research.
The time was meant for highlighting OHDSI capabilities, not testing them. The hours were meant to sharing global research, not sharing in global research.
The Observational Health Data Sciences and Informatics (OHDSI) community held a COVID-19 global, virtual study-a-thon March 26-29, believing that a network of people who valued both collaboration and open science could make a meaningful impact on the current global pandemic.
How? Nobody was quite sure in the moment, but they were confident they would figure it out.
“We chose an ambitious path and relied on our community and infrastructure to lead the way,” said Patrick Ryan. “In simple terms, efforts within our community over the past 88 months set the foundation for OHDSI’s most important and impactful 88 hours.”
(Click here for the full feature story on the OHDSI COVID-19 study-a-thon)
Largest Observational Study on Hydroxychloroquine Safety Profile Released in OHDSI Preprint
 The Observational Health Data Sciences and Informatics (OHDSI) collaboration released a preprint on preliminary findings from the largest study ever completed on the safety profile of hydroxychloroquine, a drug currently being evaluated as a potential treatment for COVID-19.
The Observational Health Data Sciences and Informatics (OHDSI) collaboration released a preprint on preliminary findings from the largest study ever completed on the safety profile of hydroxychloroquine, a drug currently being evaluated as a potential treatment for COVID-19.
Preprints are preliminary reports of work that have not been certified by peer review. They should not be relied on to guide clinical practice or health-related behavior and should not be reported in news media as established information.
The preprint is available here.
The combined short-term use of hydroxychloroquine and azithromycin resulted in nearly 60% higher rate of cardiovascular-related mortality (calHR 2.19; (1.22-3.94)) than the combined use of hydroxychloroquine and amoxicillin. While not as high, there was also an advanced risk for both chest pain/angina (calHR 1.15 (1.05-1.26)) and heart failure (calHR 1.22 (1.02-1.45)) when azithromycin was added to hydroxychloroquine treatment.
These findings were generated from an international database of more than 950,000 users of hydroxychloroquine, including approximately 320,000 who used it in combination with azithromycin.
The short-term effect of hydroxychloroquine as a treatment drug was not found to have an excess risk by itself when compared to sulfasalazine among a large set of patients (950,769 and 306,706, respectively) being treated for rheumatoid arthritis.
Patients from five different countries (Germany, Japan, Spain, the United State, and the United Kingdom) were included in this study, the first to be shared via preprint from a four-day OHDSI COVID-19 study-a-thon, which brought together a global community to design and execute observational studies to generate real-world evidence and help inform the current global pandemic.
These are preliminary findings that are currently in the peer-review process. They should not be relied on to guide clinical practice or health-related behavior and should not be reported in news media as established information.
OHDSI Collaboration Designs COVID-19 Studies For International Observational Data Network
 A four-day global collaboration within the Observational Health Data Sciences and Informatics (OHDSI) community designed and began executing studies on an international set of observational health databases (including insurance claims and electronic health records) to aid decision-making during the current COVID-19 pandemic.
A four-day global collaboration within the Observational Health Data Sciences and Informatics (OHDSI) community designed and began executing studies on an international set of observational health databases (including insurance claims and electronic health records) to aid decision-making during the current COVID-19 pandemic.
One study is the first large-scale characterization of COVID-19 patients in both the United States and Asia; six databases with COVID-19 patients located in both the U.S. and South Korea already started running data on this project, and other databases are being sought to collaborate in this network study.
The largest study ever conducted on the safety of hydroxychloroquine was designed and executed across an international set of databases. This study of more than 130,000 patients from the USA, England, Germany and South Korea focuses on the overall safety profile of hydroxychloroquine, a drug currently being evaluated as a potential treatment for COVID-19.
The third study designed the first prediction model externally validated on COVID-19 patients to support triage decisions in an effort to ‘flatten the curve’. This model, which determines which patients presenting with symptoms are most likely to require hospitalization, was developed against US data and then tested on South Korean data.
More than 330 people from 30 nations registered to collaborate in this 88-hour virtual study-a-thon, which concluded March 29 with a global presentation from multiple study leads to announce both designs and preliminary findings. Results are currently being evaluated and papers are actively being submitted to journals for peer review.
OHDSI Kicks Off International Collaborative to Generate Real-World Evidence on COVID-19 with Virtual Study-a-thon March 26-29
 The Observational Health Data Sciences and Informatics (OHDSI) international community will host a COVID-19 virtual study-a-thon this week (March 26-29) to inform healthcare decision-making in response to the current global pandemic.
The Observational Health Data Sciences and Informatics (OHDSI) international community will host a COVID-19 virtual study-a-thon this week (March 26-29) to inform healthcare decision-making in response to the current global pandemic.
More than 290 people from 29 different countries have registered for the four-day online event, which will be led by researchers from Oxford University, Erasmus Medical Center, Columbia University, UCLA, Ajou University, Janssen Research and Development, and IQVIA, with active participation across government, industry, and academia.
Held in lieu of the canceled OHDSI European Symposium, this event is structured to have two main goals: (1) to generate immediate real-world evidence on prioritized questions shared by national governments, public health agencies, health-related institutions, and community members; and (2) to design COVID-19-specific studies that can be validated and available to run when such data is available.
Cervical Cancer Risk Decreases In Users Of Copper IUDs vs. Hormonal IUDs; Research Team Seeks Community Involvement For Network Study
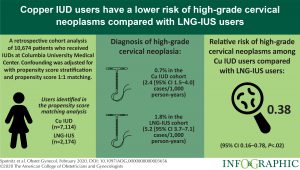 Patients who used copper intrauterine devices (Cu IUD) were found to have a lower risk of high-grade cervical neoplasms (cervical cancer) compared to users of the levonorgestrel-releasing intrauterine system (LNG-IUS), according to a Columbia study recently published in Obstetrics & Gynecology.
Patients who used copper intrauterine devices (Cu IUD) were found to have a lower risk of high-grade cervical neoplasms (cervical cancer) compared to users of the levonorgestrel-releasing intrauterine system (LNG-IUS), according to a Columbia study recently published in Obstetrics & Gynecology.
Studies from the 1980s suggested a reduced risk of cervical cancer among women who used an intrauterine contraceptive, though those studies did not differentiate between the varying types of IUDs. Furthermore, much of the data from those studies was collected prior to the availability of most hormonal IUDs (LNG-IUS).
Spotnitz noted that the research team hopes to lead a network study across other databases within the OHDSI network, which spans 19 countries, 133 unique databases converted to the OMOP CDM, and more than one billion patient records. For more information on the study, check out this forum post, or email him directly at mes2165@cumc.columbia.edu.
OHDSI Korea Symposium Page Is Live, Includes Video Of All Sessions, Photo Recap
 The 2019 OHDSI Korea Symposium took place Dec. 12-14 at the Konjiam Resort in Gwangju and attracted 330 people to the main symposium on Dec. 13. It was another great gathering of OHDSI collaborators — both veterans and those just starting on the journey — to learn from each other, present their own research, and network together.
The 2019 OHDSI Korea Symposium took place Dec. 12-14 at the Konjiam Resort in Gwangju and attracted 330 people to the main symposium on Dec. 13. It was another great gathering of OHDSI collaborators — both veterans and those just starting on the journey — to learn from each other, present their own research, and network together.
This page includes a photo gallery from the event, as well as videos all five sessions from the main symposium, as well as tutorials. Sessions included an introduction to both OHDSI and EHDEN, multiple presentations on OHDSI Community in Action, and sharing the international experience from the Asian-Pacific Region.
Recommended Diuretic Causes More Side Effects than Similar Hypertension Drug, Per Recent LEGEND Study
Chlorthalidone, the guideline-recommended diuretic for lowering blood pressure, causes more serious side effects than hydrochlorothiazide, a similarly effective diuretic, according to a recent OHDSI study. The findings, published in JAMA Internal Medicine, contrast with current treatment guidelines recommending chlorthalidone over hydrochlorothiazide.
The researchers found that patients taking chlorthalidone had nearly three times the risk of developing dangerously low levels of potassium and a greater risk of other electrolyte imbalances and kidney problems compared with those taking hydrochlorothiazide. Information from the largest individual database studied by the team revealed that 6.3% of patients treated with chlorthalidone experienced hypokalemia (low blood potassium), compared with 1.9% of patients who were treated with hydrochlorothiazide.
“Doctors prescribing chlorthalidone should monitor for certain side effects in their patients,” says George Hripcsak, MD, MS, chair and Vivian Beaumont Allen Professor of Biomedical Informatics at Columbia University and lead author of the study.
Methods Benchmark Can Aid Trust In Observational Research, Per Recent OHDSI Study
 The prevalence of electronic healthcare data allows researchers the opportunity to study the effects of medical treatments. However, confidence in the results of such observational research is typically low, for example, because different studies on the same question often produce conflicting results, even when using the same data. We need to answer the question “to what extent can we trust observational research?”
The prevalence of electronic healthcare data allows researchers the opportunity to study the effects of medical treatments. However, confidence in the results of such observational research is typically low, for example, because different studies on the same question often produce conflicting results, even when using the same data. We need to answer the question “to what extent can we trust observational research?”
Led by Martijn Schuemie, OHDSI researchers recently published “How Confident Are We About Observational Findings in Healthcare: A Benchmark Study” in the Harvard Data Science Review to tackle this important issue. This paper presents the OHDSI Methods Benchmark to evaluate five methods commonly used in observational research (new-user cohort, self-controlled cohort, case-control, case-crossover, and self-controlled case series designs) over a network of four large databases standardized to the OMOP Common Data Model.
Using both negative and positive controls (questions where the answer is known), a set of metrics and open-source software tools developed within the OHDSI community, the research team determined that most commonly used approaches to effect-estimation observational studies are falling short of expected confidence levels. Selection bias, confounding, and misspecification are among the sources of systematic error that plagues the validity of potentially important findings within the healthcare community.
OHDSI Q&A: Dani Prieto-Alhambra Discusses RA Study-A-Thon, EU Symposium
 When Dani Prieto-Alhambra discussed the Oxford Study-A-Thon at the 2019 U.S. Symposium, he introduced his talk as the “conversion of himself and 30-35 colleagues to the OMOP Common Data Model and to the OHDSI way of doing things.”
When Dani Prieto-Alhambra discussed the Oxford Study-A-Thon at the 2019 U.S. Symposium, he introduced his talk as the “conversion of himself and 30-35 colleagues to the OMOP Common Data Model and to the OHDSI way of doing things.”
After sharing the incredible research that would eventually lead to a published study in The Lancet Rheumatology, he didn’t wait long to welcome new converts to the OHDSI community. Prieto-Alhambra coordinated the 2020 Barcelona Study-A-Thon on rheumatoid arthritis (RA); you can read the OHDSI release about the event here.
He recently discussed several aspects of the study-a-thon with OHDSI.org, and he also touched on the 2020 OHDSI European Symposium, which will be held March 27-29 at Oxford. Abstracts for the Symposium are due Friday, Feb. 14; more information on abstract submission and other areas of participation is available here.
EHDEN Launches Second Open Call For SMEs
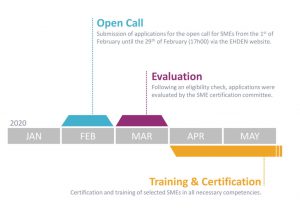 The OHDSI community has been a proud collaborator with the European Health Data & Evidence Network (EHDEN) since the EHDEN launch in 2018. An IMI 2 consortium, EHDEN looks standardize more than 100 million patient records across Europe from different geographic areas and different data sources over the coming five years. Mapping of healthcare data to the OMOP-CDM will facilitate the re-use for a variety of purposes, enhancing and accelerating research and healthcare decision-making for global benefit. To this end, EHDEN will create an SME eco-system in Europe that supports data sources and other stakeholders in mapping and using data.
The OHDSI community has been a proud collaborator with the European Health Data & Evidence Network (EHDEN) since the EHDEN launch in 2018. An IMI 2 consortium, EHDEN looks standardize more than 100 million patient records across Europe from different geographic areas and different data sources over the coming five years. Mapping of healthcare data to the OMOP-CDM will facilitate the re-use for a variety of purposes, enhancing and accelerating research and healthcare decision-making for global benefit. To this end, EHDEN will create an SME eco-system in Europe that supports data sources and other stakeholders in mapping and using data.
EHDEN recently announced that it has launched the second open call for SMEs to apply for training and certification to convert health data from various formats to the OMOP common data model. This second open call will run throughout the month of February, concluding on the 29th (17:00 CET).
If this prospect interests you, visit the EHDEN open call for SMEs page for more details and to submit your application. The EHDEN Consortium is looking forward to your potential application and to collaborate with you.
Invested Stakeholders, OHDSI Tools/Practices Drive Successful Rheumatoid Arthritis Study-A-Thon In Barcelona
 One year after a similar study-a-thon at Oxford resulted in a knee replacement study published by The Lancet Rheumatology, 40 stakeholders from across industry, academia, and health systems — representing 10 different nations and 14 observational databases — gathered to participate in a OHDSI-EHDEN Study-a-thon and run the world’s largest network studies on Rheumatoid Arthritis (RA).
One year after a similar study-a-thon at Oxford resulted in a knee replacement study published by The Lancet Rheumatology, 40 stakeholders from across industry, academia, and health systems — representing 10 different nations and 14 observational databases — gathered to participate in a OHDSI-EHDEN Study-a-thon and run the world’s largest network studies on Rheumatoid Arthritis (RA).
The Study-a-thon was held at the Barcelona Biomedical Research Park Jan. 13-17 and focused on three areas during the five-day gathering: (1) characterizing drug treatment patterns; (2) developing a population-level effect estimation, examining the comparative safety of first-line Disease Modifying Anti Rheumatic Drugs (DMARDs) for safety profiles and multiple adverse outcomes; and (3) creating a patient-level prediction analysis to determine high-risk RA patients for specific adverse outcomes. The OHDSI-EHDEN community conducted observational analyses across a secure, distributed network of electronic health records and insurance claims data, collectively representing more than 1.1 million patients with RA.
“It was an honor to collaborate with so many leaders in the battle against RA, and I truly believe we made a meaningful difference within one week,” said Patrick Ryan. “I am continually amazed at what can be accomplished when you combine invested stakeholders and high-level analytic tools in an open-science setting.”
EHDEN Academy Shows Early Success, Could Provide Educational Foundation For OHDSI Network In 2020
 As an associate professor at Erasmus Medical Center, Peter Rijnbeek appreciates the importance of a strong, effective educational program. He has been pleased with the early progress of the EHDEN Academy, a program that should provide a broad impact for the OHDSI community once it becomes publicly available in early 2020.
As an associate professor at Erasmus Medical Center, Peter Rijnbeek appreciates the importance of a strong, effective educational program. He has been pleased with the early progress of the EHDEN Academy, a program that should provide a broad impact for the OHDSI community once it becomes publicly available in early 2020.
The EHDEN Academy, an E-learning environment developed by the EHDEN (European Health Data & Evidence Network) Consortium, was initially developed to educate SMEs (Small and Medium Enterprises) about the tools and best practices used by both EHDEN and OHDSI. There are five training courses Rijnbeek and his EHDEN colleagues felt would provide a baseline of knowledge needed to certify a support network to map a growing set of European databases to the OMOP common data model.
Book Of OHDSI, Now Available In English/Korean Versions, Provides Central Knowledge Repository For Collaborators
 One memorable moment (during a day full of them) at the 2019 OHDSI U.S. Symposium came during Martijn Schuemie’s talk on best practices within the community. With a significant number of first-timers in the Bethesda North Marriott ballroom that day, Schuemie may have caused a moment of panic for some by noting all the different locations collaborators could search to follow the preferred OHDSI methods. The panic quickly turned to celebration.
One memorable moment (during a day full of them) at the 2019 OHDSI U.S. Symposium came during Martijn Schuemie’s talk on best practices within the community. With a significant number of first-timers in the Bethesda North Marriott ballroom that day, Schuemie may have caused a moment of panic for some by noting all the different locations collaborators could search to follow the preferred OHDSI methods. The panic quickly turned to celebration.
The unveiling of The Book of OHDSI at the 2019 U.S. Symposium was the culmination of months of community work, and it serves to provide the community with a central knowledge repository for all aspects of OHDSI. Twenty chapters within five sections (the OHDSI Community, Uniform Data Representation, Data Analytics, Evidence Quality, and OHDSI Studies) were written to empower any new researcher with the ability to generate real-world evidence to improve the healthcare community.
2019 Symposium Tutorials Available, Provide Education On Multiple Tools, Practices Within Community
 While OHDSI collaborators continue to seek new and innovative ways to train the growing community in the tools and best practices of the network, face-to-face tutorials remain an effective method for educating both newcomers and veterans. During the 2019 OHDSI U.S. Symposium, there were six tutorials held, and you can watch any or all of them now on the OHDSI YouTube channel.
While OHDSI collaborators continue to seek new and innovative ways to train the growing community in the tools and best practices of the network, face-to-face tutorials remain an effective method for educating both newcomers and veterans. During the 2019 OHDSI U.S. Symposium, there were six tutorials held, and you can watch any or all of them now on the OHDSI YouTube channel.
Use the headline link to get access to all six tutorials, including videos, materials, information and more.
First Korea Tutorial, OHDSI Japan Formation Highlight Exciting Asian Progress Before Korea Symposium
 The first official OHDSI Korea tutorial was held Oct. 23 in the Grand Ambassador Seoul, and it served as an important lead event for the upcoming OHDSI Korea Symposium, which takes place Dec. 12-14 at the Konjiam Resort in Gyeonggi-Do, Korea. The enthusiasm in the room was palpable, and the energy that has been building in Korea should lead to an exciting Symposium.
The first official OHDSI Korea tutorial was held Oct. 23 in the Grand Ambassador Seoul, and it served as an important lead event for the upcoming OHDSI Korea Symposium, which takes place Dec. 12-14 at the Konjiam Resort in Gyeonggi-Do, Korea. The enthusiasm in the room was palpable, and the energy that has been building in Korea should lead to an exciting Symposium.
While Korea first started working with OHDSI and the OMOP Common Data Model in 2014, workshops in the country had been limited to smaller Ajou University-sessions within hospitals. This was the first event that was formalized by OHDSI collaborators and open to all. There was a heavy morning focus on how to run a network study, which followed an OHDSI Introduction by Mui Van Zandt.
OHDSI Introduction At Georgia Tech Educates Both Students And Professor
 The first-ever OHDSI block of the CS6440 course at Georgia Tech, held over a six-week span this past fall, was both educational and inspiring, and it reinforced the strengths that have carried OHDSI from concept to major player in the real-world analytics ecosystem.
The first-ever OHDSI block of the CS6440 course at Georgia Tech, held over a six-week span this past fall, was both educational and inspiring, and it reinforced the strengths that have carried OHDSI from concept to major player in the real-world analytics ecosystem.
The professor felt it from the students, but he felt it himself as well.
Jon Duke, MD, MS, an OHDSI veteran who collaborated on the LEGEND hypertension study published recently in Lancet, is Director of Health Informatics at Georgia Tech, home of the largest computer science graduate program in the nation. When he took over the Intro to Health Informatics course in 2018, he decided to introduce population-level analytics to a rising generation of data scientists.
EHDEN Knee Replacement Study Results Published In Lancet Rheumatology; OHDSI Tools, Collaborators Helped Lead Important Study
 The IMI European Health Data & Evidence Network (EHDEN) project is pleased to announce the publication of the results of its first ‘study-a-thon’ in Lancet Rheumatology the effectiveness and safety associated with unicompartmental versus total knee replacement, a milestone after its first year.1
The IMI European Health Data & Evidence Network (EHDEN) project is pleased to announce the publication of the results of its first ‘study-a-thon’ in Lancet Rheumatology the effectiveness and safety associated with unicompartmental versus total knee replacement, a milestone after its first year.1
The choice of which type of knee replacement to recommend remains difficult for surgeons, and there remains insufficient information to inform them and patients of the best approach, dependent on the patient’s personal context.
Researchers associated with the Observational Health Data Sciences and Informatics (OHDSI) network and EHDEN met in Oxford for five days in December 2018 to design, analyze and draft a report of the study results. The resulting study emulated to the extent possible, the design of the five year Total or Partial Knee Arthroplasty Trial (TOPKAT). The study-a-thon assessed whether the efficacy results seen in the trial translated into effectiveness in real-world settings and provided further consideration of safety outcomes that were too uncommon to assess in TOPKAT.
Lancet Paper Shows Most Popular Hypertension Drug Isn’t Most Effective, Per OHDSI’s LEGEND Study
Thiazide diuretics demonstrate better effectiveness and cause fewer side effects than ACE inhibitors as first-line antihypertensive drugs, according to a report published Oct. 24 in The Lancet. The study factors insurance claim data and electronic health records from 4.9 million patients across nine observational databases, making it the most comprehensive one ever on first-line antihypertensives, and it provides additional context to the 2017 guidelines for high blood pressure treatment developed by the American College of Cardiology (ACC) and American Heart Association (AHA).
Collaborators in the Observational Health Data Sciences and Informatics (OHDSI) network produced the paper “Comprehensive comparative effectiveness and safety of first-line antihypertensive drug classes: a systematic, multinational, large-scale analysis” as part of the collaborative’s ongoing Large-Scale Evidence Generation and Evaluation across a Network of Databases (LEGEND) project, which applies high-level analytics to perform observational research on hundreds of millions of patient records within OHDSI’s international database network.
OHDSI researchers believe LEGEND will continue to significantly enhance how real-world evidence is used to study important healthcare questions that impact millions of patients worldwide.
Symposium Research Will Be Virtually Unveiled During #OHDSISocialShowcase
 There were more than 80 research highlights presented during the Collaborator Showcase at the 2019 OHDSI U.S. Symposium. For those who couldn’t attend, or who want to check out all the OHDSI innovations over the last year, you’ll have your chance on the OHDSI Twitter and LinkedIn platforms. Each weekday, a different poster will be highlighted with an individual URL. You’ll be able to check out the poster, as well as other materials the author may have included (abstract, software demo, lightning talk, etc.).
There were more than 80 research highlights presented during the Collaborator Showcase at the 2019 OHDSI U.S. Symposium. For those who couldn’t attend, or who want to check out all the OHDSI innovations over the last year, you’ll have your chance on the OHDSI Twitter and LinkedIn platforms. Each weekday, a different poster will be highlighted with an individual URL. You’ll be able to check out the poster, as well as other materials the author may have included (abstract, software demo, lightning talk, etc.).
Book of OHDSI Introduced At Symposium As Central Respository For Current, Potential Collaborators
 Martijn Schuemie, PhD, took the stage at the 2019 OHDSI U.S. Symposium and laid out a collection of internet locations where potential collaborators could learn about the tools and best practices developed within the community. To an audience that included about 200 first-time attendees, it must have been a daunting moment.
Martijn Schuemie, PhD, took the stage at the 2019 OHDSI U.S. Symposium and laid out a collection of internet locations where potential collaborators could learn about the tools and best practices developed within the community. To an audience that included about 200 first-time attendees, it must have been a daunting moment.
That feeling wouldn’t last long, as Schuemie followed by reaching under a white cover and pulling out the first version of the Book of OHDSI, the product of a year-long collaborative effort within the community to provide the best documentation for all aspects of OHDSI. Twenty chapters within five sections (the OHDSI Community, Uniform Data Representation, Data Analytics, Evidence Quality, and OHDSI Studies) were written to empower any new researcher with the ability to generate real-world evidence to improve the healthcare community.
For those who didn’t attend the Symposium, the Book of OHDSI is available here as HTML, as well as EPUB and PDF (click the small download icon at the top). Anybody who wants an actual copy of the book can order it through Amazon at cost price.
 Check Out Our All-Encompassing 2019 Symposium Recap Page!
Check Out Our All-Encompassing 2019 Symposium Recap Page!
The fifth annual OHDSI Symposium was a tremendous success. From the insightful talks and impressive poster presentations on Monday to the spectacular Women in Real-World Analytics Leadership Forum and the six tutorials, there is no shortage of important material that is now available from this event (including videos of all speeches and tutorials, as well as slides from the presentations). The page also includes video and photo recaps from the weekend, as well as the virtual collaborator showcase, which highlights more than 80 posters and software demos from the event.
A Look Inside What Is Coming at the 2019 OHDSI Symposium
Using real-world evidence to meaningfully impact the healthcare community will be the prevailing theme Sept. 15-17 during the 5th annual OHDSI U.S. Symposium. Collaborators from around the world will discuss both the direction of the OHDSI community, as well as some of its most important research achievements of the past year, in the highlight event on the OHDSI calendar.
The symposium takes place at the Bethesda North Marriott Hotel & Conference Center, and for the first time, it will include a Women in Real-World Analytics Leadership Forum, hosted Sunday night by the Women of OHDSI. This free event, which is open to all symposium attendees (you can RSVP here), will feature four prominent leaders in the real-world analytics community (Noémie Elhadad, Violanda Grigorescu, Janet Woodcock, and Joanne Waldstreicher), each of whom will share thoughts on their own journey, where they see this emerging discipline headed, and how OHDSI collaborators can improve healthcare in the future.
OHDSI FYI: How To Start A New Working Group
There has been interest recently in developing new working groups within the OHDSI community, but many have wondered what it takes to actually start a new working group. This was addressed previously in an OHDSI forum post, but we wanted to share the steps with you again.
An OHDSI working group represents a group of OHDSI collaborators who hold regular meetings with the purpose of developing shared solutions to tackle a common problem or address a knowledge/technology gap. A group of collaborators aiming to complete a network research study can be considered a study working group and are encouraged to follow these guidelines.
PheValuator Paper Highlights Potential For Reliable Phenotype Evaluation In Future Research
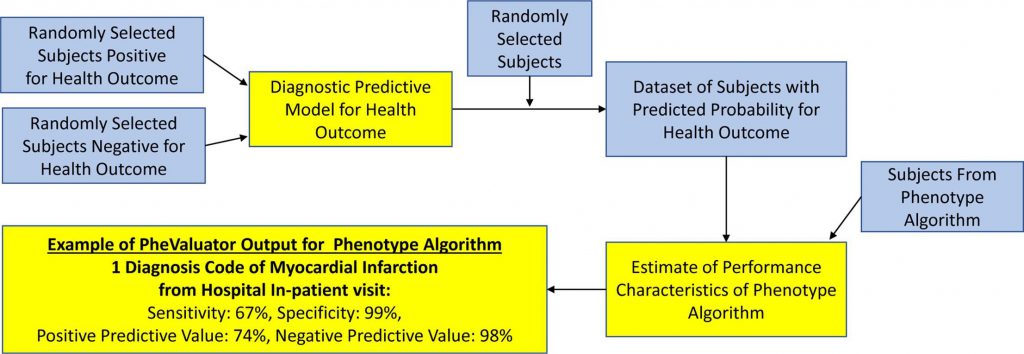 Constructing phenotype algorithms (PAs) is a primary method for both defining diseases and identifying subjects at risk for disease in observational research. While the role of PAs is crucial for effective, reproducible research, the ability to complete detailed PA evaluations has traditionally been limited due to both cost and efficiency.
Constructing phenotype algorithms (PAs) is a primary method for both defining diseases and identifying subjects at risk for disease in observational research. While the role of PAs is crucial for effective, reproducible research, the ability to complete detailed PA evaluations has traditionally been limited due to both cost and efficiency.
Lead author Joel Swerdel provided a potential solution to this challenge in PheValuator: Development and evaluation of a phenotype algorithm evaluator, published in the latest issue of the Journal of Biomedical Informatics. Utilizing tools within the OHDSI Network, the research team developed a method that showed promise for reliable phenotype evaluation without reliance on manual review of patient data.
Phenotype Sharing Feasibility Through OMOP Demonstrated In Recent JBI Paper
 Traditional methods of identifying phenotypes over varied networks of electronic health record (EHR) databases is challenging. The recently published “Facilitating Phenotype Transfer Using A Common Data Model” paper in the Journal of Biomedical Informatics demonstrated success in creating a systematic process for sharing disease definitions—known as phenotypes—across a network using the Observational Health Data Sciences and Informatics (OHDSI) OMOP Common Data Model, which could lead to dramatic improvements in the ability to study diseases in the future.
Traditional methods of identifying phenotypes over varied networks of electronic health record (EHR) databases is challenging. The recently published “Facilitating Phenotype Transfer Using A Common Data Model” paper in the Journal of Biomedical Informatics demonstrated success in creating a systematic process for sharing disease definitions—known as phenotypes—across a network using the Observational Health Data Sciences and Informatics (OHDSI) OMOP Common Data Model, which could lead to dramatic improvements in the ability to study diseases in the future.
George Hripcsak, MD, MS, the co-PI of the OHDSI Coordinating Center at Columbia University, served as lead author for a paper that demonstrated an efficient alternative to phenotype sharing that allows for rapid exchange and execution across different medical centers, improving the speed and reproducibility of the research process.
China Symposium, Tutorials Welcome Potential Collaborators To OHDSI Community
The annual OHDSI China Symposium, which took place June 27-29 at Shanghai Jiaotong University, reinforced the impressive potential of the OHDSI network via the collaboration of multiple, motivated stakeholders.
Attendees of the symposium included experts and scholars from major universities in China, medical information-related practitioners of various medical and health institutions, medical-related scientific research personnel, and workers interested in big data in the pharmaceutical industry.
The symposium led off with a pair of keynote addresses, including one entitled “FEEDERNET: Evolution of Distributed Research Network in Korea” by OHDSI collaborator Rae Woong Park. Park is a top advocate for the development of OHDSI in Korea, and his presentation helped demonstrate the unique possibilities of the network.
OHDSI Provides Oxford Tutorial, Leads One-Day Study That Posts Impressive Results

OHDSI collaborated with the Oxford Summer School session for a tutorial on tools and a one-day study.
The boundless potential to create real-world evidence through OHDSI was demonstrated for a second time in as many weeks, as collaborators from both sides of the Atlantic met in England during the Real World Epidemiology: Oxford Summer School session.
Peter Rijnbeek and Patrick Ryan led the June 27 session on the OMOP common data model, OHDSI, and the analytical use case of patient-level prediction.
Rijnbeek and Ryan supported the mission of OHDSI collaborator and Oxford professor Dani Prieto-Alhambra. The previous week, Rijnbeek and Ryan supported OHDSI collaborators Iannis Drakos and Ismail Gögenur during a three-day seminar for the Denmark Center for Surgical Sciences (CSS).
“It was great to have OHDSI join our summer school,” Prieto-Alhambra said. “Forty people’s jaws dropped whilst learning what can be achieved through open science, community and a common data model!”