- Who We Are
- Updates & News
- Standards
- Software Tools
- Network Studies
- Community Forums
- Education
- New To OHDSI?
- Community Calls
- Past Events
- Workgroups
- 2023 ‘Our Journey’ Annual Report
- This Week In OHDSI
- Support & Sponsorship
- CBER Best Seminars
- 2024 Europe Symposium
- 2024 Global Symposium
- Github
- YouTube
- Newsletters
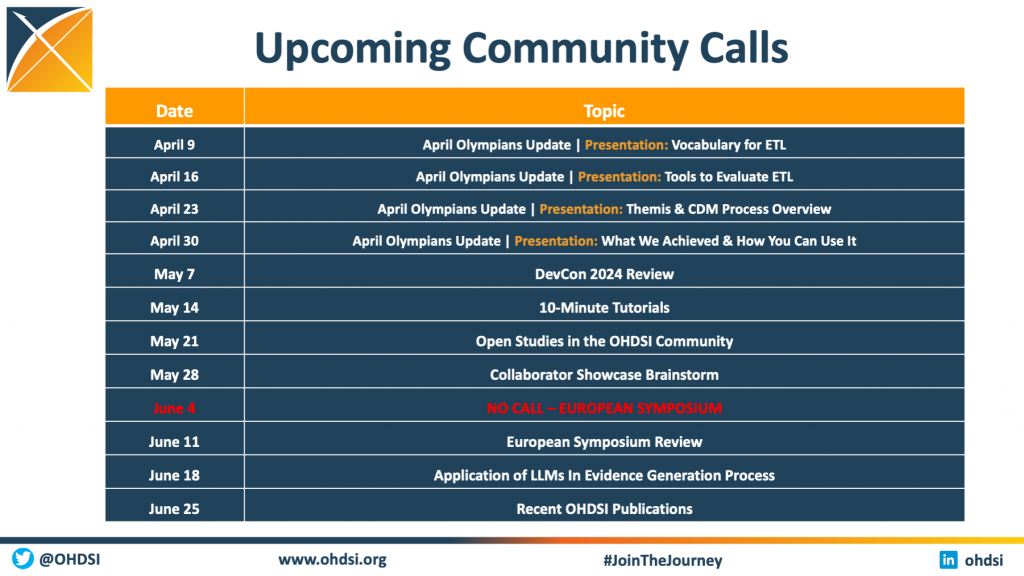
OHDSI Community Calls
Everybody is invited to the weekly OHDSI community call, which takes place each Tuesday at 11 am ET. These calls are meant to inform and engage our community through a variety of call formats, including community presentations, workgroup updates, breakout sessions, publication announcements, newcomer-focused sessions, and more. The upcoming schedule is available to the right.
Videos, slides and weekly updates from this year’s calls are available below. Presentations from the 2023, 2022 and 2021 community calls are also available.
Weekly Recordings & Updates
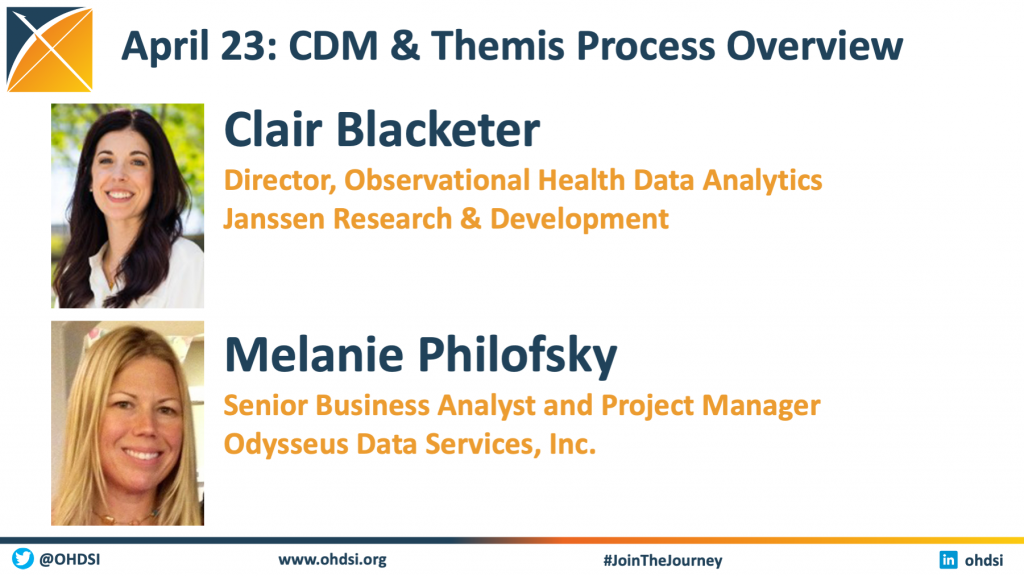 The April 23 community call focused on the CDM and Themis Process. This call provided historical and current information about both the CDM and Themis workgroups, including their mission and processes. Presentations were made by our April Olympians co-leads:
The April 23 community call focused on the CDM and Themis Process. This call provided historical and current information about both the CDM and Themis workgroups, including their mission and processes. Presentations were made by our April Olympians co-leads:
– Clair Blacketer, Director, Observational Health Data Analytics, Janssen Research & Development
– Melanie Philofsky, Senior Business Analyst and Project Manager, Odysseus Data Services, Inc.
This session also included our fourth update on the April Olympians community activity. You can watch the full presentation below.
Community Updates
• Congratulations to the team of Roger Ward, Christine Mary Hallinan, David Ormiston-Smith, Christine Chidgey, and Dougie Boyle on the publication of The OMOP common data model in Australian primary care data: Building a quality research ready harmonised dataset in PLOS One.
• Congratulations to the team of Christian Gulden, Philipp Macho, Ines Reinecke, Cosima Strantz, Hans-Ulrich Prokosch, and Romina Blasini on the publication of recruIT: A cloud-native clinical trial recruitment support system based on Health Level 7 Fast Healthcare Interoperability Resources (HL7 FHIR) and the Observational Medical Outcomes Partnership Common Data Model (OMOP CDM) in Computers in Biology and Medicine.
• Congratulations to the team of Giorgio Gandaglia, Francesco Pellegrino, Asieh Golozar, Bertrand De Meulder, Thomas Abbott, Ariel Achtman, Muhammad Imran Omar, Thamir Alshammari, Carlos Areia, Alex Asiimwe, Katharina Beyer, Anders Bjartell, Riccardo Campi, Philip Cornford, Thomas Falconer, Qi Feng, Mengchun Gong, Ronald Herrera, Nigel Hughes, Tim Hulsen, Adam Kinnaird, Lana Y.H. Lai, Gianluca Maresca, Nicolas Mottet, Marek Oja, Peter Prinsen, Christian Reich, Sebastiaan Remmers, Monique J. Roobol, Vasileios Sakalis, Sarah Seager, Emma J. Smith, Robert Snijder, Carl Steinbeisser, Nicolas H. Thurin, Ayman Hijazy, Kees van Bochove, Roderick C.N. Van den Bergh, Mieke Van Hemelrijck, Peter-Paul Willemse, Andrew E. Williams, Nazanin Zounemat Kermani, Susan Evans-Axelsson, Alberto Briganti, James N’Dow, on behalf of the PIONEER Consortium on the publication of Clinical Characterization of Patients Diagnosed with Prostate Cancer and Undergoing Conservative Management: A PIONEER Analysis Based on Big Data in European Urology.
• Applications are now being accepted for the 2024 Maternal Health Data Science Fellowship, which is designed to empower clinical investigators to leverage emerging technologies for improved maternal and neonatal care while reducing morbidity and mortality. The program, which will include the components of career development, practice and networking, will train clinical investigators in observational research methods to enable them to conduct reproducible research and generate real-world evidence. More information, including application details, are now available, and the deadline to apply is May 15, 2024.
 • The third annual OHDSI DevCon will be held virtually on Friday, April 26, from 9 am-3 pm ET. Join leaders from our Open-Source Community for a day to both welcome and inform both new and veteran developers within the OHDSI Community. More details on the agenda will be posted when available.
• The third annual OHDSI DevCon will be held virtually on Friday, April 26, from 9 am-3 pm ET. Join leaders from our Open-Source Community for a day to both welcome and inform both new and veteran developers within the OHDSI Community. More details on the agenda will be posted when available.
• Registration is now OPEN for the 2024 OHDSI Europe Symposium, which will be held June 1-3 in Rotterdam, Netherlands. There will be tutorials and workshops June 1-2 at the Erasmus University Medical Center, and the main conference will be held Monday, June 3, on the Steam Ship Rotterdam. Please visit the event homepage for more information and registration details.
2024 OHDSI Global Symposium
• Registration is now open for the 2024 Global Symposium, which will be held Oct. 22-24 at the Hyatt Regency Hotel in New Brunswick, N.J., USA. The three-day event will feature tutorials on Day 1, plenaries and the collaborator showcase on Day 2, and workgroup activities on Day 3.
• Day 1 will open with a single tutorial in the morning: An Introduction to the Journey from Data to Evidence using OHDSI. There will be four advanced tutorials during the afternoon: An Introduction to the Journey from Data to Evidence using OHDSI; Developing and Evaluating Your Extract, Transform, Load (ETL) Process to the OMOP Common Data Model; So, You Think You Want To Run an OHDSI Network Study?; and Using the OHDSI Standardized Vocabularies for Research. You can select your tutorials during the registration process.
• Collaborator Showcase submissions are now being accepted, and all details about the event are available here. Submissions are due by Friday, June 21, at 8 pm ET. Notifications of acceptance will be sent out by Tuesday, Aug. 20.
OHDSI Social Showcase
• Research from the 2023 Global Symposium Collaborator Showcase can be viewed on the Global Symposium Showcase page. Research is also being shared daily on OHDSI’s LinkedIn, Twitter/X and Instagram feeds as part of the #OHDSISocialShowcase. Below are posters (with study leads) that are featured this week:
Monday — Enhancing Data Quality Management: Introducing Capture and Cleanse Modes to the Data Quality Dashboard (Frank DeFalco)
Tuesday — Making OMOP Happen: An Implementation Science Approach (Maya Younoszai)
Wednesday — Evaluation of Study Execution using Large-Scale Analytics: A Machine Learning Approach to Assess Pre-Exposure Prophylaxis (PrEP) Utilization in the Real-World (Nag Mani)
Thursday — Validation and Comparison of Frailty Indexes: An OHDSI Network Study (Chen Yanover)
Friday — Broadsea 3.0: “BROADening the ohdSEA” (Ajit Londhe)
Job Openings
• Dani Prieto-Alhambra recently shared an opening for two Research Assistants in Health Data Sciences to join the Pharmaco- and Device epidemiology research group at the Botnar Research Centre, NDORMS, University of Oxford. In this role, you will contribute to the programming of analytical pipelines for the analysis of routinely collected data mapped to the OMOP Common Data Model. You will analyze real world data to address regulatory questions related to the prevalence/incidence of disease, use of medicines/vaccines, and the risks or benefits of medicines/vaccines or devices. You will prepare analytical packages to run a number of pre-specified analyses, contribute to wider project planning, including ideas for new research projects and gather, analyze, and present scientific data from a variety of sources. The application deadline is May 10. More information and an application link are available here.
• Alex Asiimwe shared an opening for a Director, RWE – Data Science at Gilead. As a RWE Data Scientist (OMOP/OHDSI), you will play a crucial role in designing, implementing, and maintaining healthcare data solutions using the OHDSI framework. This position offers an exciting opportunity to contribute to advancements in health informatics and research.
• Linying Zhang shared openings for both a Postdoc and a Senior Data Analyst at the Washington University School of Medicine in St. Louis. Successful candidates will work on causal machine learning and responsible AI for reliable real-world evidence generation. Interested applicants should send a CV and cover letter to linyingz@wustl.edu.
Slides
CDM Process | Themis Process | Community Updates
Video Presentation
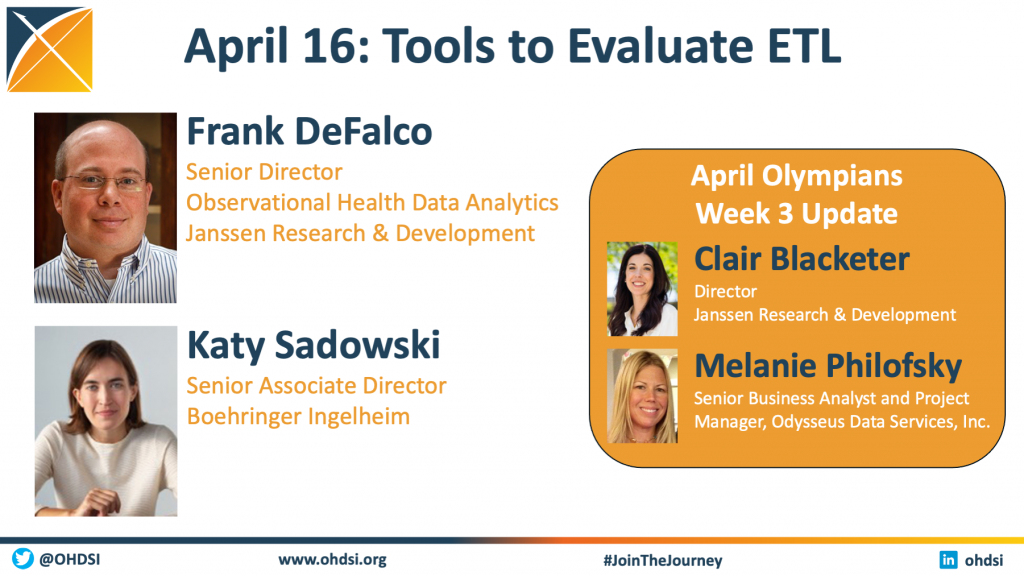 The April 16 community call focused on Tools to Evaluate ETL. We welcomed two community leaders to lead our main session:
The April 16 community call focused on Tools to Evaluate ETL. We welcomed two community leaders to lead our main session:
– Frank DeFalco, Senior Director, Observational Health Data Analytics at Janssen Research & Development
– Katy Sadowski, Senior Associate Director, Boehringer Ingelheim
This session also included our third update on the April Olympians community activity. Both presentations are available below.
Community Updates
• Congratulations to the team of Nhung TH Trinh, Annika M Jödicke, Martí Català, Núria Mercadé-Besora, Saeed Hayati, Angela Lupattelli, Daniel Prieto-Alhambra, and Hedvig ME Nordeng on the publication of Effectiveness of COVID-19 vaccines to prevent long COVID: data from Norway in The Lancet Respiratory Medicine.
• Congratulations to the team of Guy Tsafnat, Rachel Dunscombe, Davera Gabriel, Grahame Grieve, and Christian Reich on the publication of Converge or Collide? Making Sense of a Plethora of Open Data Standards in Health Care in the Journal of Medical Internet Research.
• Congratulations to the team of Ailbhe Lawlor, Carol Lin, Juan Gómez Rivas, Laura Ibáñez, Pablo Abad López, Peter-Paul Willemse, Muhammad Imran Omar, Sebastiaan Remmers, Philip Cornford, Pawel Rajwa, Rossella Nicoletti, Giorgio Gandaglia, Jeremy Yuen-Chun Teoh, Jesús Moreno Sierra, Asieh Golozar, Anders Bjartell, Susan Evans-Axelsson, James N’Dow, Jihong Zong, Maria J. Ribal, Monique J. Roobol, Mieke Van Hemelrijck, Katharina Beyer, on behalf of the PIONEER Consortium on the publication of Predictive Models for Assessing Patients’ Response to Treatment in Metastatic Prostate Cancer: A Systematic Review in European Urology Open Science.
• Applications are now being accepted for the 2024 Maternal Health Data Science Fellowship, which is designed to empower clinical investigators to leverage emerging technologies for improved maternal and neonatal care while reducing morbidity and mortality. The program, which will include the components of career development, practice and networking, will train clinical investigators in observational research methods to enable them to conduct reproducible research and generate real-world evidence. More information, including application details, are now available, and the deadline to apply is May 15, 2024.
• The CBER BEST Initiative Seminar Series returns Wednesday, April 17 (11 am – 12 pm ET) as 2021 Titan Award honoree Yong Chen presents his research on“Real-World Effectiveness of BNT162b2 Against Infection and Severe Diseases in Children and Adolescents: causal inference under misclassification in treatment status.” This series is open to anybody: Calendar invite to CBER BEST Seminar.
 • The third annual OHDSI DevCon will be held virtually on Friday, April 26, from 9 am-3 pm ET. Join leaders from our Open-Source Community for a day to both welcome and inform both new and veteran developers within the OHDSI Community. More details on the agenda will be posted when available.
• The third annual OHDSI DevCon will be held virtually on Friday, April 26, from 9 am-3 pm ET. Join leaders from our Open-Source Community for a day to both welcome and inform both new and veteran developers within the OHDSI Community. More details on the agenda will be posted when available.
• The 2024 OHDSI Global Symposium will be held Oct. 22-24 at the Hyatt Regency Hotel in New Brunswick, NJ. The tentative symposium format will feature tutorials on Oct. 22, plenaries and the collaborator showcase on Oct. 23, and workgroup activities on Oct. 24. Registration has not opened yet.
• Registration is now OPEN for the 2024 OHDSI Europe Symposium, which will be held June 1-3 in Rotterdam, Netherlands. There will be tutorials and workshops June 1-2 at the Erasmus University Medical Center, and the main conference will be held Monday, June 3, on the Steam Ship Rotterdam. Please visit the event homepage for more information and registration details.
• Atif Adam announced an opportunity for collaboration around a new network study focusing on Acute ST-Elevation Myocardial Infarction (STEMI). The study intends to deepen the understanding of STEMI patients’ characteristics and identify incidence rates across multiple real-world data datasets. More details and information on how to get involved are available within the forum post.
• Research from the 2023 Global Symposium Collaborator Showcase can be viewed on the Global Symposium Showcase page. Research is also being shared daily on OHDSI’s LinkedIn, Twitter/X and Instagram feeds as part of the #OHDSISocialShowcase. Below are posters (with study leads) that are featured this week:
Monday — Augmenting the National COVID Cohort Collaborative (N3C) Dataset with Medicare and Medicaid (CMS) Data, Secure and Deidentified Clinical Dataset (Stephanie Hong)
Tuesday — The Feasibility of Clinical Quality Language (CQL) Based Digital Quality Measures (dQMs) Implementation to OMOP CDM (Emir Amaro Syailendra)
Wednesday — Using Cohort Diagnostics to Assess the Phenotypic Data Quality in All of Us Research Program (Lina Sulieman)
Thursday — Demonstration of the OHDSI phenotype library (Gowtham Rao)
Friday — Large variety Country size RWD data-lake (Guy Livne)
Job Openings
• Alex Asiimwe shared an opening for a Director, RWE – Data Science at Gilead. As a RWE Data Scientist (OMOP/OHDSI), you will play a crucial role in designing, implementing, and maintaining healthcare data solutions using the OHDSI framework. This position offers an exciting opportunity to contribute to advancements in health informatics and research.
• Linying Zhang shared openings for both a Postdoc and a Senior Data Analyst at the Washington University School of Medicine in St. Louis. Successful candidates will work on causal machine learning and responsible AI for reliable real-world evidence generation. Interested applicants should send a CV and cover letter to linyingz@wustl.edu.
Slides
ACHILLES/ARES | DataQualityDashboard | Community Updates
Video Presentation
Tools to Evaluate ETL
April Olympians Update
 The April 9 community call focused on Vocabulary Techniques for ETL. This call also included our second April Olympians update (Clair Blacketer and Melanie Philofsky), as well as the first OKR presentation from the newly formed Rehabilitation Workgroup (Ruud Selles and Esther Janssen).
The April 9 community call focused on Vocabulary Techniques for ETL. This call also included our second April Olympians update (Clair Blacketer and Melanie Philofsky), as well as the first OKR presentation from the newly formed Rehabilitation Workgroup (Ruud Selles and Esther Janssen).
We welcomed four community leaders to present on Vocabulary Techiques for ETL:
– Dmitry Dymshyts, Associate Director, Janssen Research & Development
– Tanya Skugarevskaya, Vocabulary Team, Odysseus Data Services, Inc.
– Anna Ostropolets, Associate Director, Janssen Research & Development
– Alexander Davydov, Director, Lead of Medical Ontologies, Odysseus Data Services, Inc.
Community Updates
• Congratulations to the team of Pawel Rajwa, Angelika Borkowetz, Thomas Abbott, Andrea Alberti, Anders Bjartell, James T. Brash, Riccardo Campi, Andrew Chilelli, Mitchell Conover, Niculae Constantinovici, Eleanor Davies, Bertrand De Meulder, Sherrine Eid, Mauro Gacci, Asieh Golozar, Haroon Hafeez, Samiul Haque, Ayman Hijazy, Tim Hulsen, Andreas Josefsson, Sara Khalid, Raivo Kolde, Daniel Kotik, Samu Kurki, Mark Lambrecht, Chi-Ho Leung, Julia Moreno, Rossella Nicoletti, Daan Nieboer, Marek Oja, Soundarya Palanisamy, Peter Prinsen, Christian Reich, Giulio Raffaele Resta, Maria J Ribal, Juan Gómez Rivas, Emma Smith, Robert Snijder, Carl Steinbeisser, Frederik Vandenberghe, Philip Cornford, Susan Evans-Axelsson, James N’Dow, and Peter-Paul M Willemse on the publication of Research Protocol for an Observational Health Data Analysis on the Adverse Events of Systemic Treatment in Patients with Metastatic Hormone-sensitive Prostate Cancer: Big Data Analytics Using the PIONEER Platform in European Urology Open Science.
• The CBER BEST Initiative Seminar Series returns Wednesday, April 17 (11 am – 12 pm ET) as 2021 Titan Award honoree Yong Chen presents his research on“Real-World Effectiveness of BNT162b2 Against Infection and Severe Diseases in Children and Adolescents: causal inference under misclassification in treatment status.” This series is open to anybody: Calendar invite to CBER BEST Seminar.
• The latest OHDSI newsletter is now available. This newsletter includes information on the recent standardized vocabularies release, a preview of the April Olympians collaborative activity, the latest collaborator spotlight on Melanie Philofsky, the monthly videocast, links to the nine published studies from the OHDSI community in March, and plenty more. If you don’t receive the newsletter in your inbox, you can subscribe here.
• Melanie Philofsky is a Senior Business & Data Analyst with Odysseus Data Services, Inc. She is responsible for the harmonization of various healthcare data sources into the OMOP Common Data Model to support research endeavors. Her areas of expertise include clinical informatics, data analysis, data quality, ETL conversions, EHR data, the OMOP CDM and data modeling of new domains. Melanie was the 2022 Titan Award honoree for Contributions in Data Standards. In the latest edition of the Collaborator Spotlight, she discusses her career journey, her work with the Healthcare Systems and Themis workgroups, plans for the April Olympians Collab-a-thon, and more!
• The HADES-wide release 2024Q1 has been released. This is a snapshot of the HADES packages and their dependencies, thoroughly tested and confirmed to be mutually compatible. It is intended to be a stable environment for studies and execution engines. Currently, the release is only available as an renv lock file 3, but folks are working on containers as well.
• The third annual OHDSI DevCon will be held virtually on Friday, April 26, from 9 am-3 pm ET. Join leaders from our Open-Source Community for a day to both welcome and inform both new and veteran developers within the OHDSI Community. More details on the agenda will be posted when available.
• The 2024 OHDSI Global Symposium will be held Oct. 22-24 at the Hyatt Regency Hotel in New Brunswick, NJ. The tentative symposium format will feature tutorials on Oct. 22, plenaries and the collaborator showcase on Oct. 23, and workgroup activities on Oct. 24. Registration has not opened yet.
• Registration is now OPEN for the 2024 OHDSI Europe Symposium, which will be held June 1-3 in Rotterdam, Netherlands. There will be tutorials and workshops June 1-2 at the Erasmus University Medical Center, and the main conference will be held Monday, June 3, on the Steam Ship Rotterdam. Please visit the event homepage for more information and registration details.
• Atif Adam announced an opportunity for collaboration around a new network study focusing on Acute ST-Elevation Myocardial Infarction (STEMI). The study intends to deepen the understanding of STEMI patients’ characteristics and identify incidence rates across multiple real-world data datasets. More details and information on how to get involved are available within the forum post.
• Research from the 2023 Global Symposium Collaborator Showcase can be viewed on the Global Symposium Showcase page. Research is also being shared daily on OHDSI’s LinkedIn, Twitter/X and Instagram feeds as part of the #OHDSISocialShowcase. Below are posters (with study leads) that are featured this week:
Monday — Implementing the OMOP common data model in an NHS Trust using DBT (Quinta Ashcroft)
Tuesday — Mining Data Outside the Box: Internet as a New Source for Common Data Model (Min-Gyu Kim)
Wednesday — Forecasting Daily Incidence of Respiratory Symptoms: A Comparative Study on Time Series Models using OMOP-CDM in South Korea (Min Ho An)
Thursday — Observational Research in Dentistry: A Scoping Review (Robert Koski)
Friday — Integration of Scalable Natural Language Processing to the Atlas Cohort Building Workflow (Pavan Parimi)
Job Openings
• Alex Asiimwe shared an opening for a Director, RWE – Data Science at Gilead. As a RWE Data Scientist (OMOP/OHDSI), you will play a crucial role in designing, implementing, and maintaining healthcare data solutions using the OHDSI framework. This position offers an exciting opportunity to contribute to advancements in health informatics and research.
• Linying Zhang shared openings for both a Postdoc and a Senior Data Analyst at the Washington University School of Medicine in St. Louis. Successful candidates will work on causal machine learning and responsible AI for reliable real-world evidence generation. Interested applicants should send a CV and cover letter to linyingz@wustl.edu.
Slides
Vocabulary Techniques for ETL | Future Directions in Vocabularies | Community Updates
Video Presentation
The April 2 (11 am ET) community call featured our first “April Olympians” session. Event co-leads Clair Blacketer and Melanie Philofsky provided the first update, including examples of what the ‘hunters’ and ‘writers’ will do during the month, and details on how to get involved throughout the month. Anton Ivanov also joined to discuss the Perseus ETL tool.
Community Updates
• Congratulations to the team of Kipyo Kim, Ji-Eun Kim, Jae Ho Kim, Seong Hee Ahn, Chai Young Jung, Seun Deuk Hwang, Seoung Woo Lee and Joon Ho Song on the publication of Real-world evidence of constipation and laxative use in the Korean population with chronic kidney disease from a common data model in Scientific Reports.
• Congratulations to the team of Cindy Cai, Akihiko Nishimura, Mary Bowring, Erik Westlund, Diep Tran, Jia Ng, Paul Nagy, Michael Cook, Jody-Ann McLeggon, Scott DuVall, Michael Matheny, Asieh Golozar, Anna Ostropolets, Evan Minty, Priya Desai, Fan Bu, Brian Toy, Michelle Hribar, Thomas Falconer, Linying Zhang, Laurence Lawrence-Archer, Michael Boland, Kerry Goetz, Nathan Hall, Azza Shoaibi, Jenna Reps, Anthony Sena, Clair Blacketer, Joel Swerdel, Kenar Jhaveri, Edward Lee, Zachary Gilbert, Scott Zeger, Deidra Crews, Marc Suchard, George Hripcsak, and Patrick Ryan on the publication of Similar risk of kidney failure among patients with blinding diseases who receive ranibizumab, aflibercept, and bevacizumab: an OHDSI Network Study in Ophthalmology Retina.
• Congratulations to the team of Joshua Ide, Azza Shoaibi, Kerstin Wagner, Rachel Weinstein, Kathleen E. Boyle and Andrew Myers on the publication of Patterns of Comorbidities and Prescribing and Dispensing of Non-steroidal Anti-inflammatory Drugs (NSAIDs) Among Patients with Osteoarthritis in the USA: Real-World Study in Drugs & Aging.
• Congratulations to the team of Jens Weidner, Ingmar Glauche, Ulf Manuwald, Ivana Kern, Ines Reinecke, Franziska Bathelt, Makan Amin, Fan Dong, Ulrike Rothe, and Joachim Kugler on the publication of Correlation of Socioeconomic and Environmental Factors With Incidence of Crohn Disease in Children and Adolescents: Systematic Review and Meta-Regression in JMR Public Health and Surveillance.
• Congratulations to the team of Valerie van Baalen, Eva-Maria Didden, Daniel Rosenberg, Kristina Bardenheuer, Michel van Speybroeck, and Monika Brand on the publication of Increase transparency and reproducibility of real-world evidence in rare diseases through disease-specific Federated Data Networks in Pharmacoepidemiology & Drug Safety.
• The CBER BEST Initiative Seminar Series returns Wednesday, April 17 (11 am – 12 pm ET) as 2021 Titan Award honoree Yong Chen presents his research on“Real-World Effectiveness of BNT162b2 Against Infection and Severe Diseases in Children and Adolescents: causal inference under misclassification in treatment status.” This series is open to anybody: Calendar invite to CBER BEST Seminar.
• The latest OHDSI newsletter is now available. This newsletter includes information on the recent standardized vocabularies release, a preview of the April Olympians collaborative activity, the latest collaborator spotlight on Melanie Philofsky, the monthly videocast, links to the nine published studies from the OHDSI community in March, and plenty more. If you don’t receive the newsletter in your inbox, you can subscribe here.
• Melanie Philofsky is a Senior Business & Data Analyst with Odysseus Data Services, Inc. She is responsible for the harmonization of various healthcare data sources into the OMOP Common Data Model to support research endeavors. Her areas of expertise include clinical informatics, data analysis, data quality, ETL conversions, EHR data, the OMOP CDM and data modeling of new domains. Melanie was the 2022 Titan Award honoree for Contributions in Data Standards. In the latest edition of the Collaborator Spotlight, she discusses her career journey, her work with the Healthcare Systems and Themis workgroups, plans for the April Olympians Collab-a-thon, and more!
• The third annual OHDSI DevCon will be held virtually on Friday, April 26, from 9 am-3 pm ET. Join leaders from our Open-Source Community for a day to both welcome and inform both new and veteran developers within the OHDSI Community. More details on the agenda will be posted when available.
• The 2024 OHDSI Global Symposium will be held Oct. 22-24 at the Hyatt Regency Hotel in New Brunswick, NJ. The tentative symposium format will feature tutorials on Oct. 22, plenaries and the collaborator showcase on Oct. 23, and workgroup activities on Oct. 24. Registration has not opened yet.
• Registration is now OPEN for the 2024 OHDSI Europe Symposium, which will be held June 1-3 in Rotterdam, Netherlands. There will be tutorials and workshops June 1-2 at the Erasmus University Medical Center, and the main conference will be held Monday, June 3, on the Steam Ship Rotterdam. Please visit the event homepage for more information and registration details.
• Atif Adam announced an opportunity for collaboration around a new network study focusing on Acute ST-Elevation Myocardial Infarction (STEMI). The study intends to deepen the understanding of STEMI patients’ characteristics and identify incidence rates across multiple real-world data datasets. More details and information on how to get involved are available within the forum post.
• Research from the 2023 Global Symposium Collaborator Showcase can be viewed on the Global Symposium Showcase page. Research is also being shared daily on OHDSI’s LinkedIn, Twitter/X and Instagram feeds as part of the #OHDSISocialShowcase. Below are posters (with study leads) that are featured this week:
Monday — Integrating clinical and laboratory research data using the OMOP CDM (Edward Frankenberger)
Tuesday — A new route of administration hierarchy derived from dose forms supporting standardised drug dose calculations (Theresa Burkard)
Wednesday — Bayesian Evidence Synthesis with Bias Correction (Louisa Smith)
Thursday — Save Our Sisyphus Challenge: Lessons learned from Strategus execution on the OHDSI Network (Anthony Sena)
Friday — Incorporating Real-World Data Research in Training First-Year Medical Students Using OHDSI OMOP and Atlas tools (Pavel Goriacko)
Job Openings
• Alex Asiimwe shared an opening for a Director, RWE – Data Science at Gilead. As a RWE Data Scientist (OMOP/OHDSI), you will play a crucial role in designing, implementing, and maintaining healthcare data solutions using the OHDSI framework. This position offers an exciting opportunity to contribute to advancements in health informatics and research.
• Linying Zhang shared openings for both a Postdoc and a Senior Data Analyst at the Washington University School of Medicine in St. Louis. Successful candidates will work on causal machine learning and responsible AI for reliable real-world evidence generation. Interested applicants should send a CV and cover letter to linyingz@wustl.edu.
• Nathan Hall introduced a summer internship at Johnson & Johnson for an Epidemiology UX/Web Design Intern. This internship provides a unique opportunity to merge design principles with epidemiological research, contributing to the advancement of real-world evidence applications. In this role, you will have the opportunity to blend your passion for user experience (UX) and web design with the field of epidemiology, contributing to impactful projects that enhance our ability to derive insights from health data. More information and an application link are available here.
Slides
Video Presentation
 The March 26 community call featured a session on recent publications from the OHDSI community. OHDSI collaborators have published more than 600 studies related to the OMOP CDM and/or OHDSI tools or methods, and we welcomed five lead authors to present their recent publications.
The March 26 community call featured a session on recent publications from the OHDSI community. OHDSI collaborators have published more than 600 studies related to the OMOP CDM and/or OHDSI tools or methods, and we welcomed five lead authors to present their recent publications.
1) Tathagata Bhattacharjee • University of London
INSPIRE datahub: a pan-African integrated suite of services for harmonising longitudinal population health data using OHDSI tools • Frontiers in Digital Health
2) Sulev Reisberg • University of Tartu
Transforming Estonian health data to the Observational Medical Outcomes Partnership (OMOP) Common Data Model: lessons learned • JAMIA Open
3) Fan Bu • University of Michigan
Bayesian safety surveillance with adaptive bias correction • Statistics in Medicine
4) Jen Wooyeon Park • Johns Hopkins University
Development of Medical Imaging Data Standardization for Imaging-Based Observational Research: OMOP Common Data Model Extension • Journal of Imaging Informatics in Medicine
5) Christian Reich • Odysseus
OHDSI Standardized Vocabularies—a large-scale centralized reference ontology for international data harmonization • JAMIA
Community Updates
• Congratulations to the team of Markus Haug, Marek Oja, Maarja Pajusalu, Kerli Mooses, Sulev Reisberg, Jaak Vilo, Antonio Fernández Giménez, Thomas Falconer, Ana Danilović, Filip Maljkovic, Dalia Dawoud, and Raivo Kolde on the publication of Markov modeling for cost-effectiveness using federated health data network in JAMIA.
• Congratulations to the team of Philippe Mortier, Franco Amigo, Madhav Bhargav, Susana Conde, Montse Ferrer, Oskar Flygare, Busenur Kizilaslan, Laura Latorre Moreno, Angela Leis, Miguel Angel Mayer, Víctor Pérez-Sola, Ana Portillo-Van Diest, Juan Manuel Ramírez-Anguita, Ferran Sanz, Gemma Vilagut, Jordi Alonso, Lars Mehlum, Ella Arensman, Johan Bjureberg, Manuel Pastor and Ping Qin on the publication of Developing a clinical decision support system software prototype that assists in the management of patients with self-harm in the emergency department: protocol of the PERMANENS project in BMC Psychiatry.
• The third annual OHDSI DevCon will be held virtually on Friday, April 26, from 9 am-3 pm ET. Join leaders from our Open-Source Community for a day to both welcome and inform both new and veteran developers within the OHDSI Community. More details on the agenda will be posted when available.
• The 2024 OHDSI Global Symposium will be held Oct. 22-24 at the Hyatt Regency Hotel in New Brunswick, NJ. The tentative symposium format will feature tutorials on Oct. 22, plenaries and the collaborator showcase on Oct. 23, and workgroup activities on Oct. 24. Registration has not opened yet.
• Registration is now OPEN for the 2024 OHDSI Europe Symposium, which will be held June 1-3 in Rotterdam, Netherlands. There will be tutorials and workshops June 1-2 at the Erasmus University Medical Center, and the main conference will be held Monday, June 3, on the Steam Ship Rotterdam. Please visit the event homepage for more information and registration details.
• Atif Adam announced an opportunity for collaboration around a new network study focusing on Acute ST-Elevation Myocardial Infarction (STEMI). The study intends to deepen the understanding of STEMI patients’ characteristics and identify incidence rates across multiple real-world data datasets. More details and information on how to get involved are available within the forum post.
• Research from the 2023 Global Symposium Collaborator Showcase can be viewed on the Global Symposium Showcase page. Research is also being shared daily on OHDSI’s LinkedIn, Twitter/X and Instagram feeds as part of the #OHDSISocialShowcase. Below are posters (with study leads) that are featured this week:
Monday — Synthesizing Evidence for Rare Events: a Novel Zero-Inflated Bivariate Model to Integrate Studies with Double-Zero Outcomes (Lu Li)
Tuesday — Active Safety Surveillance Using Real-world Evidence (ASSURE): An application of the Strategus package (Kevin Haynes)
Wednesday — Patient’s outcomes after endoscopic retrograde cholangiopancreatography (ERCP) using reprocessed duodenoscope accessories: a descriptive study using real-world data (Jessica Mayumi Maruyama)
Thursday — Does COVID-19 Increase Racial/Ethnic Differences in Prevalence of Post-acute Sequelae of SARS-CoV-2 infection (PASC) in Children and Adolescents? an EHR-Based Cohort from the RECOVER Program (Dazheng Zhang)
Friday — Eye Care and Vision Research Workgroup: First Year Update (Michelle Hribar)
Job Openings
• Alex Asiimwe shared an opening for a Director, RWE – Data Science at Gilead. As a RWE Data Scientist (OMOP/OHDSI), you will play a crucial role in designing, implementing, and maintaining healthcare data solutions using the OHDSI framework. This position offers an exciting opportunity to contribute to advancements in health informatics and research.
• Linying Zhang shared openings for both a Postdoc and a Senior Data Analyst at the Washington University School of Medicine in St. Louis. Successful candidates will work on causal machine learning and responsible AI for reliable real-world evidence generation. Interested applicants should send a CV and cover letter to linyingz@wustl.edu.
• Nathan Hall introduced a summer internship at Johnson & Johnson for an Epidemiology UX/Web Design Intern. This internship provides a unique opportunity to merge design principles with epidemiological research, contributing to the advancement of real-world evidence applications. In this role, you will have the opportunity to blend your passion for user experience (UX) and web design with the field of epidemiology, contributing to impactful projects that enhance our ability to derive insights from health data. More information and an application link are available here.
Slides
Bhattacharjee | Reisberg | Bu | Park | Reich | Community Updates
Videos
INSPIRE datahub: a pan-African integrated suite of services for harmonising longitudinal population health data using OHDSI tools (Bhattacharjee)
Transforming Estonian health data to the Observational Medical Outcomes Partnership (OMOP) Common Data Model: lessons learned (Reisberg)
Bayesian safety surveillance with adaptive bias correction (Bu)
Development of Medical Imaging Data Standardization for Imaging-Based Observational Research: OMOP Common Data Model Extension (Park)
OHDSI Standardized Vocabularies—a large-scale centralized reference ontology for international data harmonization (Reich)
The March 12 community call featured a session on March Madness and April Olympians. March Madness: Less than two weeks after the latest release of OHDSI Standardized Vocabularies, this session found some of the most interesting and unique concepts for a head-to-head showdown, March Madness style. Exploding head syndrome. Dragon’s Blood Extract. Collision of spacecraft with other spacecraft. And God Only Knows what else. April Olympians: Clair Blacketer provided a brief introduction to April Olympians, a month-long community activity that will focus on CDM and Themis conventions. More information and a registration link are below.
Community Updates
• As mentioned above, Clair Blacketer and Melanie Philofsky will lead a month-long community activity in April that will focus on CDM and Themis conventions. There will be five goals of this event:
– Identify all currently ratified CDM and THEMIS conventions for every CDM table and field
– Write clear documentation for each THEMIS convention
– Establish a repository for THEMIS conventions
– Update the CDM documentation to link to relevant THEMIS repository entries
– Create CDM documentation related to expansion module efforts around the community
If you would like to participate in this event, please fill out this form.
• The 2024 OHDSI Global Symposium will be held Oct. 22-24 at the Hyatt Regency Hotel in New Brunswick, NJ. The tentative symposium format will feature tutorials on Oct. 22, plenaries and the collaborator showcase on Oct. 23, and workgroup activities on Oct. 24. Registration has not opened yet.
• Registration is now OPEN for the 2024 OHDSI Europe Symposium, which will be held June 1-3 in Rotterdam, Netherlands. There will be tutorials and workshops June 1-2 at the Erasmus University Medical Center, and the main conference will be held Monday, June 3, on the Steam Ship Rotterdam. Please visit the event homepage for more information and registration details.
• Evanette Burrows shared an update about a new release to the ETL-Synthea package v2.0.0 that went live on Feb. 26. The package has been expanded to support current versions of Synthea (v3.1 and v3.2) and has a handful of other improvements and contributions from the community. Full release details are available here.
• Atif Adam announced an opportunity for collaboration around a new network study focusing on Acute ST-Elevation Myocardial Infarction (STEMI). The study intends to deepen the understanding of STEMI patients’ characteristics and identify incidence rates across multiple real-world data datasets. More details and information on how to get involved are available within the forum post.
• James Weaver, an Associate Director of Observational Health Data Analytics at Janssen Research and Development, will speak during a panel session on Current Approaches for Distributed Analysis on Thursday, March 14 (1 pm ET) during a Health Data Research Network Canada event. This will be a virtual conversation; more information and a registration link are available here.
• Research from the 2023 Global Symposium Collaborator Showcase can be viewed on the Global Symposium Showcase page. Research is also being shared daily on OHDSI’s LinkedIn, Twitter/X and Instagram feeds as part of the #OHDSISocialShowcase. Below are posters (with study leads) that are featured this week:
Monday — Bladder cancer – a quality benchmark utilizing FHIR and OMOP (Andries Clinckaert)
Tuesday — Using MONAI Pre-Trained Models for Colorectal Tissue Type Phenotyping: A Feasibility Study to Integrate Deep Learning Model Results using the Medical Extension OMOP CDM (Shijia Zhang)
Wednesday — A tool for empirically identifying and reviewing candidate comparators for Pharmacoepidemiological studies (Justin Bohn)
Thursday — The necessity of validity diagnostics when drawing causal inferences from observational data (James Weaver)
Friday — Identification of HIV positive individuals across multiple datasets (Craig Mayer)
Job Openings
• Alex Asiimwe shared an opening for a Director, RWE – Data Science at Gilead. As a RWE Data Scientist (OMOP/OHDSI), you will play a crucial role in designing, implementing, and maintaining healthcare data solutions using the OHDSI framework. This position offers an exciting opportunity to contribute to advancements in health informatics and research.
• Linying Zhang shared openings for both a Postdoc and a Senior Data Analyst at the Washington University School of Medicine in St. Louis. Successful candidates will work on causal machine learning and responsible AI for reliable real-world evidence generation. Interested applicants should send a CV and cover letter to linyingz@wustl.edu.
• Nathan Hall introduced a summer internship at Johnson & Johnson for an Epidemiology UX/Web Design Intern. This internship provides a unique opportunity to merge design principles with epidemiological research, contributing to the advancement of real-world evidence applications. In this role, you will have the opportunity to blend your passion for user experience (UX) and web design with the field of epidemiology, contributing to impactful projects that enhance our ability to derive insights from health data. More information and an application link are available here.
Slides
April Olympians | Community Updates
Videos
March Madness
April Olympians
 The March 5 community call focused on the latest vocabulary release, which was shared 29Feb2024, as well as a brief wrap-up discussion around Phenotype Phebruary 2024. The main session will be driven by leaders from our vocabulary team:
The March 5 community call focused on the latest vocabulary release, which was shared 29Feb2024, as well as a brief wrap-up discussion around Phenotype Phebruary 2024. The main session will be driven by leaders from our vocabulary team:
- Alexander Davydov, Director, Lead of Medical Ontologies • Odysseus Data Services
- Oleg Zhuk, Vocabulary Technical Lead • Odysseus Data Services
- Anna Ostropolets, Associate Director, Observational Health Data Analytics • Janssen Research and Development
Community Updates
• Congratulations to the team of Aniek F Markus, Peter R Rijnbeek, Jan A Kors, Edward Burn, Talita Duarte-Salles, Markus Haug, Chungsoo Kim, Raivo Kolde, Youngsoo Lee, Hae-Sim Park, Rae Woong Park, Daniel Prieto-Alhambra, Carlen Reyes, Jerry A Krishnan, Guy G Brusselle, and Katia MC Verhamme on the publication of Real-world treatment trajectories of adults with newly diagnosed asthma or COPD in BMJ Open Respiratory Research.
• Congratulations to the team of Behzad Naderalvojoud, Catherine Curtin, Chen Yanover, Tal El-Hay, Byungjin Choi, Rae Woong Park, Javier Gracia Tabuenca, Mary Pat Reeve, Thomas Falconer, Keith Humphreys, Steven M Asch, and Tina Hernandez-Boussard on the publication of Towards global model generalizability: independent cross-site feature evaluation for patient-level risk prediction models using the OHDSI network in JAMIA.
• Congratulations to the team of Star Liu, Asieh Golozar, Nathan Buesgens, Jody-Ann McLeggon, Adam Black, and Paul Nagy on the publication of A framework for understanding an open scientific community using automated harvesting of public artifacts in JAMIA Open.
• Congratulations to the team of Yi Chai, Kenneth K. C. Man, Hao Luo, Carmen Olga Torre, Yun Kwok Wing, Joseph F. Hayes, David P. J. Osborn, Wing Chung Chang, Xiaoyu Lin, Can Yin, Esther W. Chan, Ivan C. H. Lam, Stephen Fortin, David M. Kern, Dong Yun Lee, Rae Woong Park, Jae-Won Jang, Jing Li, Sarah Seager, Wallis C. Y. Lau, and Ian C. K. Wong on the publication of Incidence of mental health diagnoses during the COVID-19 pandemic: a multinational network study in Epidemiology and Psychiatric Sciences.
• Congratulations to the team of Quentin Marcou, Laure Berti-Equille, and Noël Novelli on the publication of Creating a computer assisted ICD coding system: Performance metric choice and use of the ICD hierarchy in the Journal of Biomedical Informatics.
• The 2024 OHDSI Global Symposium will be held Oct. 22-24 at the Hyatt Regency Hotel in New Brunswick, NJ. The tentative symposium format will feature tutorials on Oct. 22, plenaries and the collaborator showcase on Oct. 23, and workgroup activities on Oct. 24. Registration has not opened yet.
• Registration is now OPEN for the 2024 OHDSI Europe Symposium, which will be held June 1-3 in Rotterdam, Netherlands. There will be tutorials and workshops June 1-2 at the Erasmus University Medical Center, and the main conference will be held Monday, June 3, on the Steam Ship Rotterdam. Please visit the event homepage for more information and registration details.
• Ross Williams is a scientific researcher working in the group of Dr. Peter Rijnbeek at Erasmus MC, where he is part of the Health Data Science group. His main focus is creating tools and analysis methods to develop personalised medical risk prediction. His specific areas of interest are on the external validation of prediction models, net benefit assessment and techniques for temporal health data analysis. He co-leads both the Patient Level Prediction workgroup and the Early-Stage Researcher workgroup. Ross discusses his career journey, how observational data impacts prediction models, the opportunities for junior researchers in OHDSI, and plenty more in the latest edition of the Collaborator Spotlight.
• The latest edition of the OHDSI newsletter is now available. It includes links to all workgroup OKR presentations from last month, as well as updates from Phenotype Phebruary. It also includes the monthly video podcast, 11 February publications, a new Collaborator Spotlight, and more. If you don’t receive the monthly newsletter in your inbox, please subscribe here.
• James Weaver, an Associate Director of Observational Health Data Analytics at Janssen Research and Development, will speak during a panel session on Current Approaches for Distributed Analysis on Thursday, March 14 (1 pm ET) during a Health Data Research Network Canada event. This will be a virtual conversation; more information and a registration link are available here.
• Research from the 2023 Global Symposium Collaborator Showcase can be viewed on the Global Symposium Showcase page. Research is also being shared daily on OHDSI’s LinkedIn, Twitter/X and Instagram feeds as part of the #OHDSISocialShowcase. Below are posters (with study leads) that are featured this week:
Monday — Implementing a common data model in ophthalmology: Comparison of general eye examination mapping to standard OMOP concepts across two major EHR systems (Justin Quon)
Tuesday — Measuring Study Potential Through the Use of Data Diagnostics (Clair Blacketer)
Wednesday — Utilizing Graph Embeddings for Multiple Sclerosis Disease Modifying Therapy Adverse Events (Jason Patterson)
Thursday — Comorbidity Co-occurrence in Women with Endometriosis: A Retrospective Matched Cohort Study (Tamar Zelovich)
Friday — Ulysses: Introducing a workflow R package for assisting in the development of OHDSI studies (Martin Lavallee)
Job Openings
• Priya Desai shared an opening for a Biomedical Informatics Data Scientist at Stanford University who will partner with researchers and clinicians to enable effective and efficient use of data and resources available via Stanford’s research clinical data repository (STARR) including the Electronic Health Records in the OMOP Common Data Model, radiology and cardiology imaging data and associated metadata, and new data types as they get integrated along with their databases and respective cohort query tools and interfaces e.g., OHDSI ATLAS.
• Alex Asiimwe shared three openings at Gilead that could be of interest to OHDSI collaborators. There are openings for a Director, RWE – Data Science, a Director, Data Acquisition, and a Senior Director, Head of Data Office. You can learn more about each position and apply using the links above.
• Linying Zhang shared openings for both a Postdoc and a Senior Data Analyst at the Washington University School of Medicine in St. Louis. Successful candidates will work on causal machine learning and responsible AI for reliable real-world evidence generation. Interested applicants should send a CV and cover letter to linyingz@wustl.edu.
• Nathan Hall introduced a summer internship at Johnson & Johnson for an Epidemiology UX/Web Design Intern. This internship provides a unique opportunity to merge design principles with epidemiological research, contributing to the advancement of real-world evidence applications. In this role, you will have the opportunity to blend your passion for user experience (UX) and web design with the field of epidemiology, contributing to impactful projects that enhance our ability to derive insights from health data. More information and an application link are available here.
• There is an opening for an Epidemiology Graduate Intern at Johnson & Johnson. Among the responsibilities for this remote position are assisting in managing epidemiologic studies and literature reviews to characterize incidence, prevalence, risk factors, and associated comorbidities and treatment patterns for lung, head, and neck cancers across interventional oncology, contributing to the development of protocols for analyzing real-world data cohorts, such as SEER Medicare, and conducting quantitative analyses using both public and private data sources. More information and an application link are available here.
Slides
Vocabulary Release | Community Updates/Phenotype Phebruary
Videos
Vocabulary Release (Alexander Davydov, Oleg Zhuk, Anna Ostropolets)
Phenotype Phebruary (Azza Shoaibi)
Each community call during “Phenotype Phebruary” features a set of Workgroup 2024 Objectives & Key Result (OKR) announcements, as well an update from that week’s Phenotype Phebruary activities and findings. Workgroups that presented during this call were FHIR + OMOP, Health Equity, the Africa Chapter, Electronic Animal Health Records, CDM Vocabulary, Phenotype Development & Evaluation, Dentistry, Medical Imaging, Medical Devices and GIS – Geographic Information System. The Phenotype Phebruary update, led by Evan Minty and Eva-Maria Didden, reflected on findings from the first three weeks, as well as early research and critical questions around the Week 4 phenotype, pulmonary arterial hypertension.
Community Updates
• Congratulations to the team of Christine Mary Hallinan, Roger Ward, Graeme K Hart, Clair Sullivan, Nicole Pratt, Ashley Ng, Daniel Capurro, Anton Van Der Vegt, Siaw-Teng Liaw, Oliver Daly, Blanca Gallego Luxan, David Bunker and Douglas Boyle on the publication of Seamless EMR data access: Integrated governance, digital health and the OMOP-CDM in BMJ Health & Care Informatics.
• The 2024 OHDSI Global Symposium will be held Oct. 22-24 at the Hyatt Regency Hotel in New Brunswick, NJ. The tentative symposium format will feature tutorials and workshops on Oct. 22, the main conference on Oct. 23, and workgroup activities on Oct. 24. Registration has not opened yet.
• Registration is now OPEN for the 2024 OHDSI Europe Symposium, which will be held June 1-3 in Rotterdam, Netherlands. There will be tutorials and workshops June 1-2 at the Erasmus University Medical Center, and the main conference will be held Monday, June 3, on the Steam Ship Rotterdam. Please visit the event homepage for more information and registration details.
• Henrik John recently announced a new network study that he is leading with Chungsoo Kim, Jenna Reps and Egill Fridgeirsson on “Deep Learning Comparison.” The aim is to assess the value of deep learning methods over conventional methods for the development of clinical prediction models. The specific diseases under consideration are dementia in individuals over 55, lung cancer in those over 45, and bipolar disorder in patients misdiagnosed with major depressive disorder. If you would like to join this effort, please read this forum post for more information and reach out to the study leads by March 1.
• James Weaver, an Associate Director of Observational Health Data Analytics at Janssen Research and Development, will speak during a panel session on Current Approaches for Distributed Analysis on Thursday, March 14 (1 pm ET) during a Health Data Research Network Canada event. This will be a virtual conversation; more information and a registration link are available here.
• Under the leadership of Azza Shoaibi, Anna Ostropolets, Gowtham Rao and James Weaver, Phenotype Phebruary 2024 focuses on assessing consistency in phenotype definition components, phenotype representation structure, and phenotype validation methods. The month-long activity empowers OHDSI collaborators to engage with each other while advancing the science of phenotyping and gaining education and training around phenotype development and evaluation. You can check out the event homepage here.
• Research from the 2023 Global Symposium Collaborator Showcase can be viewed on the Global Symposium Showcase page. Research is also being shared daily on OHDSI’s LinkedIn, Twitter/X and Instagram feeds as part of the #OHDSISocialShowcase. Below are posters (with study leads) that are featured this week:
Monday — Brazilian administrative data for real-world research: a deterministic linkage procedure and OMOP CDM harmonization (Jessica Mayumi Maruyama)
Tuesday — Estimating model performance on external data sources from their summary statistics: a real-world benchmark (Tal El-Hay)
Wednesday — Harmonization of OMOP vaccine-related vocabularies through the Vaccine Ontology (Yuanyi Pan)
Thursday — OHDSI on Databricks: A Complete Guide to Implementing OHDSI on Databricks (John Gresh)
Friday — Antihypertensive medication use in pregnancy: A pilot OHDSI network analysis in electronic health record data (Stephanie Leonard)
Job Openings
• Alex Asiimwe shared three openings at Gilead that could be of interest to OHDSI collaborators. There are openings for a Director, RWE – Data Science, a Director, Data Acquisition, and a Senior Director, Head of Data Office. You can learn more about each position and apply using the links above.
• Linying Zhang shared openings for both a Postdoc and a Senior Data Analyst at the Washington University School of Medicine in St. Louis. Successful candidates will work on causal machine learning and responsible AI for reliable real-world evidence generation. Interested applicants should send a CV and cover letter to linyingz@wustl.edu.
• Nathan Hall introduced a summer internship at Johnson & Johnson for an Epidemiology UX/Web Design Intern. This internship provides a unique opportunity to merge design principles with epidemiological research, contributing to the advancement of real-world evidence applications. In this role, you will have the opportunity to blend your passion for user experience (UX) and web design with the field of epidemiology, contributing to impactful projects that enhance our ability to derive insights from health data. More information and an application link are available here.
• There is an opening for an Epidemiology Graduate Intern at Johnson & Johnson. Among the responsibilities for this remote position are assisting in managing epidemiologic studies and literature reviews to characterize incidence, prevalence, risk factors, and associated comorbidities and treatment patterns for lung, head, and neck cancers across interventional oncology, contributing to the development of protocols for analyzing real-world data cohorts, such as SEER Medicare, and conducting quantitative analyses using both public and private data sources. More information and an application link are available here.
• The University of North Carolina Chapel Hill CTSA is hiring a Research Informatics Specialist. This is a remote position (US only, with mostly East Coast hours). This person will join a skilled and highly collaborative team of data analysts, software developers, and data scientists within our CTSA. The core purpose of this position is to support the All of Us Center for Linkage and Acquisition of Data project. We are looking for folks with SQL and health care and/or claims data experience, especially OMOP.
• There is an opening for a Data Steward position at the EBMT. Among the responsibilities is the design, implementation and testing of new data collection processes including data collection forms (DCFs) development, as well as the mapping of new items from DCFs to the OMOP CDM.
Slides
Phenotype Phebruary | Workgroup OKRs + Community Updates
Videos
Workgroup Updates (FHIR + OMOP, Health Equity, the Africa Chapter, Electronic Animal Health Records, CDM Vocabulary, Phenotype Development & Evaluation, Dentistry, Medical Imaging, Medical Devices and GIS – Geographic Information System)
Phenotype Phebruary Update #4
Each community call during “Phenotype Phebruary” features a set of Workgroup 2024 Objectives & Key Result (OKR) announcements, as well an update from that week’s Phenotype Phebruary activities and findings. Workgroups that presented during this call were Themis, Healthcare Systems, Generative AI and Foundational Models, Oncology, Vaccine Vocabulary, Patient-Level Prediction (PLP), ATLAS, Open-Source Community, Psychiatry, and Natural Language Processing (NLP). The Phenotype Phebruary update, led by Anna Ostropolets and Jamie Weaver, reflected on findings from the first two weeks, as well as early research and critical questions around the Week 3 phenotype, major depressive disorder.
Community Updates
• Congratulations to the team of Martin Boeker, Daniela Zöller, Romina Blasini, Philipp Macho, Sven Helfer, Max Behrens, Hans-Ulrich Prokosch and Christian Gulden on the publication of Effectiveness of IT-supported patient recruitment: study protocol for an interrupted time series study at ten German university hospitals in Trials.
• Congratulations to the team of Moshe Zisser and Dvir Aran on the publication of Transformer-based time-to-event prediction for chronic kidney disease deterioration in JAMIA.
• The 2024 OHDSI Global Symposium will be held Oct. 22-24 at the Hyatt Regency Hotel in New Brunswick, NJ. The tentative symposium format will feature tutorials and workshops on Oct. 22, the main conference on Oct. 23, and workgroup activities on Oct. 24. Registration has not opened yet.
• Registration is now OPEN for the 2024 OHDSI Europe Symposium, which will be held June 1-3 in Rotterdam, Netherlands. There will be tutorials and workshops June 1-2 at the Erasmus University Medical Center, and the main conference will be held Monday, June 3, on the Steam Ship Rotterdam. Please visit the event homepage for more information and registration details.
• Henrik John recently announced a new network study that he is leading with Chungsoo Kim, Jenna Reps and Egill Fridgeirsson on “Deep Learning Comparison.” The aim is to assess the value of deep learning methods over conventional methods for the development of clinical prediction models. The specific diseases under consideration are dementia in individuals over 55, lung cancer in those over 45, and bipolar disorder in patients misdiagnosed with major depressive disorder. If you would like to join this effort, please read this forum post for more information and reach out to the study leads by March 1.
• James Weaver, an Associate Director of Observational Health Data Analytics at Janssen Research and Development, will speak during a panel session on Current Approaches for Distributed Analysis on Thursday, March 14 (1 pm ET) during a Health Data Research Network Canada event. This will be a virtual conversation; more information and a registration link are available here.
• Under the leadership of Azza Shoaibi, Anna Ostropolets, Gowtham Rao and James Weaver, Phenotype Phebruary 2024 focuses on assessing consistency in phenotype definition components, phenotype representation structure, and phenotype validation methods. The month-long activity empowers OHDSI collaborators to engage with each other while advancing the science of phenotyping and gaining education and training around phenotype development and evaluation. You can check out the event homepage here.
• Research from the 2023 Global Symposium Collaborator Showcase can be viewed on the Global Symposium Showcase page. Research is also being shared daily on OHDSI’s LinkedIn, Twitter/X and Instagram feeds as part of the #OHDSISocialShowcase. Below are posters (with study leads) that are featured this week:
Monday — Challenges and opportunities in adopting OMOP-CDM in Brazilian healthcare: a report from Hospital Israelita Albert Einstein (Maria Abrahao)
Tuesday — Developing a pregnancy algorithm in ATLAS: Applying start date offset (Rupa Makadia)
Wednesday — Creating parsimonious patient-level prediction models using feature selection (Aniek Markus)
Thursday — From Complexity to Clarity: Reproducible and Scalable Phenotype Development and application of LLM in a support role (Asieh Golozar)
Friday — Establishing and Operating the OHDSI Dentistry Workgroup: A Model for Other Disciplines (Danielle Boyce)
Job Openings
• Alex Asiimwe shared three openings at Gilead that could be of interest to OHDSI collaborators. There are openings for a Director, RWE – Data Science, a Director, Data Acquisition, and a Senior Director, Head of Data Office. You can learn more about each position and apply using the links above.
• Linying Zhang shared openings for both a Postdoc and a Senior Data Analyst at the Washington University School of Medicine in St. Louis. Successful candidates will work on causal machine learning and responsible AI for reliable real-world evidence generation. Interested applicants should send a CV and cover letter to linyingz@wustl.edu.
• Nathan Hall introduced a summer internship at Johnson & Johnson for an Epidemiology UX/Web Design Intern. This internship provides a unique opportunity to merge design principles with epidemiological research, contributing to the advancement of real-world evidence applications. In this role, you will have the opportunity to blend your passion for user experience (UX) and web design with the field of epidemiology, contributing to impactful projects that enhance our ability to derive insights from health data. More information and an application link are available here.
• There is an opening for an Epidemiology Graduate Intern at Johnson & Johnson. Among the responsibilities for this remote position are assisting in managing epidemiologic studies and literature reviews to characterize incidence, prevalence, risk factors, and associated comorbidities and treatment patterns for lung, head, and neck cancers across interventional oncology, contributing to the development of protocols for analyzing real-world data cohorts, such as SEER Medicare, and conducting quantitative analyses using both public and private data sources. More information and an application link are available here.
• The University of North Carolina Chapel Hill CTSA is hiring a Research Informatics Specialist. This is a remote position (US only, with mostly East Coast hours). This person will join a skilled and highly collaborative team of data analysts, software developers, and data scientists within our CTSA. The core purpose of this position is to support the All of Us Center for Linkage and Acquisition of Data project. We are looking for folks with SQL and health care and/or claims data experience, especially OMOP.
• There is an opening for a Data Steward position at the EBMT. Among the responsibilities is the design, implementation and testing of new data collection processes including data collection forms (DCFs) development, as well as the mapping of new items from DCFs to the OMOP CDM.
Slides
Phenotype Phebruary | Workgroup OKRs + Community Updates
Videos
Workgroup Updates (Themis, Healthcare Systems, Generative AI and Analytics in Healthcare (GAIA), Oncology, Vaccine Vocabulary, Patient-Level Prediction, ATLAS, Open Source Community, Psychiatry, and NLP)
Phenotype Phebruary Update #3
Each community call during “Phenotype Phebruary” features a set of Workgroup 2024 Objectives & Key Result (OKR) announcements, as well an update from that week’s Phenotype Phebruary activities and findings. Workgroups that presented during this call were Common Data Model, Network Data Quality, Asia-Pacific (APAC), Industry, Eye Care & Vision Research, and Surgery & Perioperative Medicine. The Phenotype Phebruary update focused on the findings from the Week 1 phenotype, Alzheimer’s Disease, as well as early research and critical questions around the Week 2 phenotype, non-small cell and small cell lung cancer.
Community Updates
• Congratulations to the team of Xinyuan Zhang, Yixue Feng, Fang Li, Jin Ding, Danyal Tahseen, Ezekiel Hinojosa, Yong Chen, and Cui Tao on the publication of Evaluating MedDRA-to-ICD terminology mappings in BMC Medical Informatics and Decision Making.
• Congratulations to the team of Tathagata Bhattacharjee, Sylvia Kiwuwa-Muyingo, Chifundo Kanjala, Molulaqhooa L Maoyi, David Amadi, Michael Ochola, Damazo Kadengye, Arofan Gregory, Agnes Kiragga, Amelia Taylor, Jay Greenfield, Emma Slaymaker, Jim Todd, and the INSPIRE Network on the publication of INSPIRE datahub: a pan-African integrated suite of services for harmonising longitudinal population health data using OHDSI tools in Frontiers in Digital Health.
• Registration is now OPEN for the 2024 OHDSI Europe Symposium, which will be held June 1-3 in Rotterdam, Netherlands. There will be tutorials and workshops June 1-2 at the Erasmus University Medical Center, and the main conference will be held Monday, June 3, on the Steam Ship Rotterdam. Please visit the event homepage for more information and registration details.
• If you are interested in joining the Scientific Review Committee for the 2024 Global Symposium, you can sign up now. The first meeting for the Scientific Review Committee will be held March 7.
• Henrik John recently announced a new network study that he is leading with Chungsoo Kim, Jenna Reps and Egill Fridgeirsson on “Deep Learning Comparison.” The aim is to assess the value of deep learning methods over conventional methods for the development of clinical prediction models. The specific diseases under consideration are dementia in individuals over 55, lung cancer in those over 45, and bipolar disorder in patients misdiagnosed with major depressive disorder. If you would like to join this effort, please read this forum post for more information and reach out to the study leads by March 1.
• James Weaver, an Associate Director of Observational Health Data Analytics at Janssen Research and Development, will speak during a panel session on Current Approaches for Distributed Analysis on Thursday, March 14 (1 pm ET) during a Health Data Research Network Canada event. This will be a virtual conversation; more information and a registration link are available here.
• Under the leadership of Azza Shoaibi, Anna Ostropolets, Gowtham Rao and James Weaver, Phenotype Phebruary 2024 focuses on assessing consistency in phenotype definition components, phenotype representation structure, and phenotype validation methods. The month-long activity empowers OHDSI collaborators to engage with each other while advancing the science of phenotyping and gaining education and training around phenotype development and evaluation. You can check out the event homepage here.
• Research from the 2023 Global Symposium Collaborator Showcase can be viewed on the Global Symposium Showcase page. Research is also being shared daily on OHDSI’s LinkedIn, Twitter/X and Instagram feeds as part of the #OHDSISocialShowcase. Below are posters (with study leads) that are featured this week:
Monday — Toward a General-Purpose Geography-Focused OHDSI Infrastructure (Kyle Zollo-Venecek)
Tuesday — The Development and Validation of an Individual-Level Socioeconomic Deprivation Index (ISDI) with OMOP in the NIH’s All of Us Data Network (Nripendra Acharya)
Wednesday — Incorporating measurement values into patient-level prediction with missing entries: a feasibility study (Xiaoyu Wang)
Thursday — The Use of the Julia Programming Language for Global Health Informatics and Observational Health Research (Jacob Zelko)
Friday — Analyzing a Tabloid Headline with Real-World Data: A Summer Intern’s Investigation (Delia Harms)
Job Openings
• Alex Asiimwe shared three openings at Gilead that could be of interest to OHDSI collaborators. There are openings for a Director, RWE – Data Science, a Director, Data Acquisition, and a Senior Director, Head of Data Office. You can learn more about each position and apply using the links above.
• Linying Zhang shared openings for both a Postdoc and a Senior Data Analyst at the Washington University School of Medicine in St. Louis. Successful candidates will work on causal machine learning and responsible AI for reliable real-world evidence generation. Interested applicants should send a CV and cover letter to linyingz@wustl.edu.
• Nathan Hall introduced a summer internship at Johnson & Johnson for an Epidemiology UX/Web Design Intern. This internship provides a unique opportunity to merge design principles with epidemiological research, contributing to the advancement of real-world evidence applications. In this role, you will have the opportunity to blend your passion for user experience (UX) and web design with the field of epidemiology, contributing to impactful projects that enhance our ability to derive insights from health data. More information and an application link are available here.
• The University of North Carolina Chapel Hill CTSA is hiring a Research Informatics Specialist. This is a remote position (US only, with mostly East Coast hours). This person will join a skilled and highly collaborative team of data analysts, software developers, and data scientists within our CTSA. The core purpose of this position is to support the All of Us Center for Linkage and Acquisition of Data project. We are looking for folks with SQL and health care and/or claims data experience, especially OMOP.
• There is an opening for a Data Steward position at the EBMT. Among the responsibilities is the design, implementation and testing of new data collection processes including data collection forms (DCFs) development, as well as the mapping of new items from DCFs to the OMOP CDM.
Slides
Phenotype Phebruary Update | Workgroup OKRs + Community Updates
Videos
Workgroup Updates (Common Data Model, Network Data Quality, Asia-Pacific (APAC), Industry, Eye Care & Vision Research, and Surgery & Perioperative Medicine)
Phenotype Phebruary Update #2
Each community call during “Phenotype Phebruary” features a set of Workgroup 2024 Objectives & Key Result (OKR) announcements, as well an update from that week’s Phenotype Phebruary activities and findings; the Week 1 phenotype focus was Alzheimer’s Disease. Workgroups that presented during this call were Methods Research, HADES, Perinatal and Reproductive Health, Registry, and the Steering Group.
Community Updates
• If you are interested in joining the Scientific Review Committee for the 2024 Global Symposium, you can sign up now. The first meeting for the Scientific Review Committee will be held March 7.
• Collaborators from both the Columbia University Department of Biomedical Informatics and the Johnson & Johnson Observational Health Data Analytics held a three-day studyathon this past weekend with a focus on women’s health initiatives, specifically endometriosis and polycystic ovary syndrome.
• Kerry Goetz is the Associate Director for the National Eye Institute’s Office of Data Science and Health Informatics at the US National Institutes of Health. In this capacity she is responsible for advancing data management and sharing strategies to make NEI data FAIR (Fully AI-Ready & Findable, Accessible, Interoperable, and Reusable). Kerry co-leads the Eye Care and Vision Research Observational Health Data Sciences and Informatics Working Group. She discusses her career journey, evidence gaps around vision research, how OHDSI impacts her PhD journey, and more in the latest collaborator spotlight.
• The latest edition of The Journey newsletter is now available. It includes details on Phenotype Phebruary, reflections on where OHDSI can go together in 2024, the latest OHDSI videocast, and more community updates. It also includes links to 17 published studies that came out of the OHDSI community in January. If you don’t receive the monthly newsletter in your email, you can subscribe here.
• February community calls will focus on both Phenotype Phebruary updates and 2024 OKR announcements by our various workgroups. All workgroup leads/representatives should sign up for one of the upcoming community calls using this link.
• Research from the 2023 Global Symposium Collaborator Showcase can be viewed on the Global Symposium Showcase page. Research is also being shared daily on OHDSI’s LinkedIn, Twitter/X and Instagram feeds as part of the #OHDSISocialShowcase. Below are posters (with study leads) that are featured this week:
Monday — FinOMOP – a population-based data network (Javier Gracia-Tabuenca)
Tuesday — Operational Definition of Adrenal diseases: Enhancing Precision and Reproducibility in Observational Data (Suhyun Kim)
Wednesday — Impact of concomitant use of proton pump inhibitors and clopidogrel on cardiovascular adverse outcomes – A multicenter study using common data model (Seonji Kim)
Thursday — Leveraging the OMOP Common Data Model to Support Distributed Health Equity Research (Sarah Gasman)
Friday — Validating a clinical informatics consulting service using negative control reference sets (Michael Jackson)
HADES Development Updates
• Martijn Schuemie announced the release of CohortMethod 5.2.1. The most important changes are updating the Capr function calls in the vignettes (the old code was no longer working), and CohortMethod now asks if you want to delete old files when you call runCmAnalyses() using an existing folder but different analysis settings than before.
• Ger Inberg announced the release of FeatureExtraction 3.4.0. It contains mainly bugfixes and furthermore the ‘cohortId’ argument in exported functions has been deprecated, one should use ‘cohortIds’ instead.
Job Openings
• Alex Asiimwe shared three openings at Gilead that could be of interest to OHDSI collaborators. There are openings for a Director, RWE – Data Science, a Director, Data Acquisition, and a Senior Director, Head of Data Office. You can learn more about each position and apply using the links above.
• Linying Zhang shared openings for both a Postdoc and a Senior Data Analyst at the Washington University School of Medicine in St. Louis. Successful candidates will work on causal machine learning and responsible AI for reliable real-world evidence generation. Interested applicants should send a CV and cover letter to linyingz@wustl.edu.
• Nathan Hall introduced a summer internship at Johnson & Johnson for an Epidemiology UX/Web Design Intern. This internship provides a unique opportunity to merge design principles with epidemiological research, contributing to the advancement of real-world evidence applications. In this role, you will have the opportunity to blend your passion for user experience (UX) and web design with the field of epidemiology, contributing to impactful projects that enhance our ability to derive insights from health data. More information and an application link are available here.
• The University of North Carolina Chapel Hill CTSA is hiring a Research Informatics Specialist. This is a remote position (US only, with mostly East Coast hours). This person will join a skilled and highly collaborative team of data analysts, software developers, and data scientists within our CTSA. The core purpose of this position is to support the All of Us Center for Linkage and Acquisition of Data project. We are looking for folks with SQL and health care and/or claims data experience, especially OMOP.
• There is an opening for a Data Steward position at the EBMT. Among the responsibilities is the design, implementation and testing of new data collection processes including data collection forms (DCFs) development, as well as the mapping of new items from DCFs to the OMOP CDM.
Slides
Phenotype Phebruary Update | Workgroup OKRs + Community Updates
Videos
Workgroup Updates (Methods Research, HADES, Perinatal and Reproductive Health, Registry, Steering Group)
Phenotype Phebruary Update #1
 The third installment of Phenotype Phebruary is approaching, and the leadership team provided an overview of the initiative, its importance in research, and how this version of Phenotype Phebruary will take place in the OHDSI community. This talk also included a “Phenotype 101” session, as well as a community vote on four phenotypes to be focused on during the month. The selections were Alzheimer’s, pulmonary hypertension, major depression disorder and prostate cancer. This session was led by:
The third installment of Phenotype Phebruary is approaching, and the leadership team provided an overview of the initiative, its importance in research, and how this version of Phenotype Phebruary will take place in the OHDSI community. This talk also included a “Phenotype 101” session, as well as a community vote on four phenotypes to be focused on during the month. The selections were Alzheimer’s, pulmonary hypertension, major depression disorder and prostate cancer. This session was led by:
• Azza Shoaibi – Director, Observational Health Data Analytics, Janssen Research and Development
• Jamie Weaver – Associate Director, Observational Health Data Analytics, Janssen Research and Development
• Anna Ostropolets – Associate Director, Observational Health Data Analytics, Janssen Research and Development
Patrick Ryan and Asieh Golozar also provided comments. You can sign up to take part in Phenotype Phebruary, and you can follow updates via the OHDSI forums.
Community Updates
• Congratulations to the team of Soobeen Seol, Jimyung Park, Chungsoo Kim, Dong Yun Lee, and Rae Woong Park on the publication of RHEA: Real-World Observational Health Data Exploration Application in Volume 310 of Studies in Health Technology and Informatics.
• Congratulations to the team of Sujin Gan, Chungsoo Kim, Dong Yun Lee, and Rae Woong Park on the publication of Prediction Models for Readmission Using Home Healthcare Notes and OMOP-CDM in Volume 310 of Studies in Health Technology and Informatics.
• Congratulations to the team of Scott L. DuVall, Craig G. Parker, Amanda R. Shields, Patrick R. Alba, Julie A. Lynch, Michael E. Matheny, and Aaron W.C. Kamauu on the publication of Toward Real-World Reproducibility: Verifying Value Sets for Clinical Research in Volume 310 of Studies in Health Technology and Informatics.
• Congratulations to the team of Piper Ranallo, Bronwyn Southwell, Christopher Tignanelli, Steven G. Johnson, Richard Krueger, Tess Sevareid-Groth, Adam Carvel, and Genevieve B. Melton on the publication of Promoting Learning Health System Cycles by Optimizing EHR Data Clinical Concept Encoding Processes in Volume 310 of Studies in Health Technology and Informatics.
• Congratulations to the team of ChulHyoung Park, Sang Jun Park, Da Yun Lee, Seng Chan You, Kihwang Lee, and Rae Woong Park on the publication of Multi-Institutional Collaborative Research Using Ophthalmic Medical Image Data Standardized by Radiology Common Data Model (R-CDM) in Volume 310 of Studies in Health Technology and Informatics.
• Congratulations to the team of Seol Whan Oh, Soo Jeong Ko, Yun Seon Im, Surin Jung, Bo Yeon Choi, Jae Yoon Kim, Sunghyeon Park, Wona Choi, and In Young Choi on the publication of Development of Integrated Data Quality Management System for Observational Medical Outcomes Partnership Common Data Model in Volume 310 of Studies in Health Technology and Informatics.
• Congratulations to the team of Martijn Schuemie, Jenna Reps, Adam Black, Frank Defalco, Lee Evans, Egill Fridgeirsson, James P. Gilbert, Chris Knoll, Martin Lavallee, Gowtham A. Rao, Peter Rijnbeek, Katy Sadowski, Anthony Sena, Joel Swerdel, Ross D. Williams, and Marc Suchard on the publication of Health-Analytics Data to Evidence Suite (HADES): Open-Source Software for Observational Research in Volume 310 of Studies in Health Technology and Informatics.
• Congratulations to the team of Jiyun Cha, Eun Kyoung Ahn, Young-Heum Yoon, and Man Young Park on the publication of Feasibility of Applying the OMOP Common Data Model to Traditional Eastern Asian Medicine Dataset in Volume 310 of Studies in Health Technology and Informatics.
• Congratulations to the team of Najia Ahmadi, Quang Vu Nguyen, Martin Sedlmayr, and Markus Wolfien on the publication of A comparative patient-level prediction study in OMOP CDM: applicative potential and insights from synthetic data in Scientific Reports.
• February community calls will focus on both Phenotype Phebruary updates and 2024 OKR announcements by our various workgroups. All workgroup leads/representatives should sign up for one of the upcoming community calls using this link.
• Research from the 2023 Global Symposium Collaborator Showcase can be viewed on the Global Symposium Showcase page. Research is also being shared daily on OHDSI’s LinkedIn, Twitter/X and Instagram feeds as part of the #OHDSISocialShowcase. Below are posters (with study leads) that are featured this week:
Monday — Transforming the Optum® Enriched Oncology module to OMOP CDM (Dmitry Dymshyts)
Tuesday — Community Contribution to the OHDSI Vocabularies: moving towards collaborative shared resource (Oleg Zhuk)
Wednesday — Evaluating confounding adjustment when sample size is small (Martijn Schuemie)
Thursday — Using a Continuous Quality Improvement (CQI) Approach for Gap Analysis of OHDSI/ATLAS as An Enterprise Self-Service Analytics Platform by Academic Medical Centers (Selvin Soby)
Friday — Risk of aortic aneurysm or dissection following exposure to fluoroquinolone antibiotics (Jack Jenetzki)
HADES Development Updates
• Martijn Schuemie announced the release of SelfControlledCaseSeries 5.1.1. This contains a fix of a minor bug introduced in 5.1.0.
Job Openings
• Linying Zhang shared openings for both a Postdoc and a Senior Data Analyst at the Washington University School of Medicine in St. Louis. Successful candidates will work on causal machine learning and responsible AI for reliable real-world evidence generation. Interested applicants should send a CV and cover letter to linyingz@wustl.edu.
• The University of North Carolina Chapel Hill CTSA is hiring a Research Informatics Specialist. This is a remote position (US only, with mostly East Coast hours). This person will join a skilled and highly collaborative team of data analysts, software developers, and data scientists within our CTSA. The core purpose of this position is to support the All of Us Center for Linkage and Acquisition of Data project. We are looking for folks with SQL and health care and/or claims data experience, especially OMOP.
• There is an opening for a Data Steward position at the EBMT. Among the responsibilities is the design, implementation and testing of new data collection processes including data collection forms (DCFs) development, as well as the mapping of new items from DCFs to the OMOP CDM.
Slides
Introduction to Phenotype Phebruary | Community Updates
Video Presentation
 The Jan. 23 community call looked at the recent UK Study-A-Thon, held last November at Saint Hilda’s College. The event focused on the use of fluoroquinolones across geographies and over time, as well as on the epidemiology and characterization of rectal prolapse and rectopexy. At the end of an intense week, the team generated three draft manuscripts almost ready for submission, and at least four conference abstracts were in the making.
The Jan. 23 community call looked at the recent UK Study-A-Thon, held last November at Saint Hilda’s College. The event focused on the use of fluoroquinolones across geographies and over time, as well as on the epidemiology and characterization of rectal prolapse and rectopexy. At the end of an intense week, the team generated three draft manuscripts almost ready for submission, and at least four conference abstracts were in the making.
Community Updates
• Congratulations to the team of Xu Zuo, Yujia Zhou, Jon Duke, George Hripcsak, Nigam Shah, Juan Banda, Ruth Reeves, Timothy Miller, Lemuel Waitman, Karthik Natarajan, and Hua Xu on the publication of Standardizing Multi-site Clinical Note Titles to LOINC Document Ontology: A Transformer-based Approach in the AMIA Annual Symposium Proceedings.
• Congratulations to the team of Huzaifa Khan, Abu Saleh Mohammad Mosa, Vyshnavi Paka, Md Kamruz Zaman Rana, Vasanthi Mandhadi, Soliman Islam, Hua Xu, James C McClay, Sraboni Sarker, Praveen Rao, and Lemuel Waitman on the publication of Mapping Clinical Documents to the Logical Observation Identifiers, Names and Codes (LOINC) Document Ontology using Electronic Health Record Systems Structured Metadata in the AMIA Annual Symposium Proceedings.
• Congratulations to the team of Joel Swerdel and Mitchell Conover on the publication of Comparing broad and narrow phenotype algorithms: differences in performance characteristics and immortal time incurred in the Journal of Pharmacy & Pharmaceutical Sciences.
• Registration is open for the 2024 Oxford Summer School: Real world evidence using the OMOP Common Data Model, which will be held June 17-21, 2024 at the University of Oxford. The Real World Evidence Summer School will provide participants with the tools and concepts necessary to plan and execute Real World Evidence studies, with a focus on the use of the OMOP common data model. Learn more about the program here.
• Research from the 2023 Global Symposium Collaborator Showcase can be viewed on the Global Symposium Showcase page. Research is also being shared daily on OHDSI’s LinkedIn, Twitter/X and Instagram feeds as part of the #OHDSISocialShowcase. Below are posters (with study leads) that are featured this week:
Monday — Mapping of Critical Care EHR Flowsheet data to the OMOP CDM via SSSOM (Polina Talapova)
Tuesday — Paving the way to estimate daily dose in OMOP CDM for Drug Utilisation Studies in DARWIN EU® (Theresa Burkard)
Wednesday — Generating Synthetic Electronic Health Records in OMOP using GPT (Chao Peng)
Thursday — Comparing concepts extracted from clinical Dutch text to conditions in the structured data (Tom Seinen)
Friday — Finding a constrained number of predictor phenotypes for multiple outcome prediction (Jenna Repa)
Job Openings
• The University of North Carolina Chapel Hill CTSA is hiring a Research Informatics Specialist. This is a remote position (US only, with mostly East Coast hours). This person will join a skilled and highly collaborative team of data analysts, software developers, and data scientists within our CTSA. The core purpose of this position is to support the All of Us Center for Linkage and Acquisition of Data project. We are looking for folks with SQL and health care and/or claims data experience, especially OMOP.
• There is an opening for a Data Steward position at the EBMT. Among the responsibilities is the design, implementation and testing of new data collection processes including data collection forms (DCFs) development, as well as the mapping of new items from DCFs to the OMOP CDM.
Slides
MHRA and the use of RWE | Use of systemic fluoroquinolones in primary care and hospital settings in the UK: a drug utilisation study | Rectopexy & the search for devices | Community Updates
Video Presentation
0:00 – Daniel Prieto-Alhambra – Professor of Pharmaco- and Device Epidemiology, University of Oxford (Introduction)
1:28 – Katherine Donegan – Head of Epidemiology, MHRA (MHRA and the use of RWE)
13:40 – Annika Jodicke – Senior Researcher in Pharmacoepidemiology, University of Oxford (Use of systemic fluoroquinolones in primary care and hospital settings in the UK: a drug utilisation study)
28:52 – Jennifer Lane – NIHR Clinical Lecturer in Trauma and Orthopaedic Surgery, Barts Bone and Joint Health, Queen Mary University of London (Rectopexy & the search for devices)
The Jan. 16 community call featured multiple unrecorded breakout session meant to stimulate future collaboration opportunities throughout the community. The video below highlights the updates shared at the beginning of the call, including a brief presentation on a recent community publication, Real-World Effectiveness of BNT162b2 Against Infection and Severe Diseases in Children and Adolescents.
Community Updates
• Congratulations to the team of Qiong Wu, Jiayi Tong, Bingyu Zhang, Dazheng Zhang, Jiajie Chen, Yuqing Lei, Yiwen Lu, Yudong Wang, Lu Li, Yishan Shen, Jie Xu, L. Charles Bailey, Jiang Bian, Dimitri A. Christakis, Megan L. Fitzgerald, Kathryn Hirabayashi, Ravi Jhaveri, Alka Khaitan, Tianchen Lyu, Suchitra Rao, Hanieh Razzaghi, Hayden T. Schwenk, Fei Wang, Margot I. Gage Witvliet, Eric J. Tchetgen Tchetgen, Jeffrey S. Morris, Christopher B. Forrest, and Yong Chen on the publication of Real-World Effectiveness of BNT162b2 Against Infection and Severe Diseases in Children and Adolescents in Annals of Internal Medicine.
• Congratulations to the team of Martí Català, Núria Mercadé-Besora, Raivo Kolde, Nhung T H Trinh, Elena Roel, Edward Burn, Trishna Rathod-Mistry, Kristin Kostka, Wai Yi Man, Antonella Delmestri, Prof Hedvig M E Nordeng, Anneli Uusküla, Talita Duarte-Salles, Daniel Prieto-Alhambra, and Annika M Jödicke on the publication of The effectiveness of COVID-19 vaccines to prevent long COVID symptoms: staggered cohort study of data from the UK, Spain, and Estonia in The Lancet Respiratory Medicine.
• Anna Ostropolets will lead the next edition of the CBER Best Seminar Seminar series, which will be held Wednesday, Jan. 17, at 11 am ET. Anna will lead a session entitled “KEEPER: Standardized structured data from electronic health records as an alternative to chart review for case adjudication and phenotype evaluation” which will be virtual and available to anybody. More information and the registration link are available here.
• Chungsoo Kim is a PhD candidate in the Department of Biomedical Informatics at Ajou University College of Medicine. Since joining OHDSI in 2019, he has participated in and led several research projects at OHDSI. He currently participates in OHDSI working groups, including PatientLevelPrediction and the APAC group. He also served as a tutorial instructor for the 2019 OHDSI Korea International Symposium. Chungsoo discusses his research focuses, his involvement in the OHDSI community, the growth of OHDSI around the Asia-Pacific region, and plenty more in the latest Collaborator Spotlight.
• Research from the 2023 Global Symposium Collaborator Showcase can be viewed on the Global Symposium Showcase page. Research is also being shared daily on OHDSI’s LinkedIn, Twitter/X and Instagram feeds as part of the #OHDSISocialShowcase. Below are posters (with study leads) that are featured this week:
Monday — Development of psychiatric common data model (P-CDM) leveraging psychiatric scales (Dong Yun Lee)
Tuesday — Integrating large language models and real-world evidence into an automated drug indication taxonomy development workflow (Yilu Fang)
Wednesday — Making OHDSI Tooling accessible to Researchers and Students in a HIPAA Compliant Platform (Hannah Morgan-Cooper)
Thursday — Trend in Prescription Pattern in Heart Failure Medications (Septi Melisa)
Friday — PheMIME: An Interactive Web App and Knowledge Base for Phenome-Wide Multi-Institutional Multimorbidity Analysis (Siwei Zhang)
Job Openings
• There is an opening for a Data Steward position at the EBMT. Among the responsibilities is the design, implementation and testing of new data collection processes including data collection forms (DCFs) development, as well as the mapping of new items from DCFs to the OMOP CDM.
Slides
Video Presentation
 Happy New Year! Patrick Ryan led the first community call of the year for a discussion on what OHDSI can achieve together in 2024. You can watch the recording or check out the slides below.
Happy New Year! Patrick Ryan led the first community call of the year for a discussion on what OHDSI can achieve together in 2024. You can watch the recording or check out the slides below.
Community Updates
• Congratulations to the team of Yoon Jin Choi, Tae Jun Kim, Chang Seok Bang, Yong Kang Lee, Moon Won Lee, Su Youn Nam, Woon Geon Shin, and Seung In Seo on the publication of Changing trends and characteristics of peptic ulcer disease: A multicenter study from 2010 to 2019 in Korea in the World Journal of Gastroenterology.
• Congratulations to the team of Pierre Heudel, Hugo Crochet, Thierry Durand, Philippe Zrounba, and JeanYves Blay on the publication of From data strategy to implementation to advance cancer research and cancer care: A French comprehensive cancer center experience in PLOS Digital Health.
• Congratulations to the team of Cynthia Yang, Egill Fridgeirsson, Jan Kors, Jenna Reps and Peter Rijnbeek on the publication of Impact of random oversampling and random undersampling on the performance of prediction models developed using observational health data in the Journal of Big Data.
• Congratulations to the team of Christian Reich, Anna Ostropolets, Patrick Ryan, Peter Rijnbeek, Martijn Schuemie, Alexander Davydov, Dmitry Dymshyts, and George Hripcsak on the publication of OHDSI Standardized Vocabularies-a large-scale centralized reference ontology for international data harmonization in JAMIA.
• Congratulations to the team of Qiong Wu, Jiayi Tong, Bingyu Zhang, Dazheng Zhang, Jiajie Chen, Yuqing Lei, Yiwen Lu, Yudong Wang, Lu Li, Yishan Shen, Jie Xu, L. Charles Bailey, Jiang Bian, Dimitri A. Christakis, Megan L. Fitzgerald, Kathryn Hirabayashi, Ravi Jhaveri, Alka Khaitan, Tianchen Lyu, Suchitra Rao, Hanieh Razzaghi, Hayden T. Schwenk, Fei Wang, Margot I. Gage Witvliet, Eric J. Tchetgen Tchetgen, Jeffrey S. Morris, Christopher B. Forrest, and Yong Chen on the publication of Real-World Effectiveness of BNT162b2 Against Infection and Severe Diseases in Children and Adolescents in Annals of Internal Medicine.
• Chungsoo Kim is a PhD candidate in the Department of Biomedical Informatics at Ajou University College of Medicine. Since joining OHDSI in 2019, he has participated in and led several research projects at OHDSI. He currently participates in OHDSI working groups, including PatientLevelPrediction and the APAC group. He also served as a tutorial instructor for the 2019 OHDSI Korea International Symposium. Chungsoo discusses his research focuses, his involvement in the OHDSI community, the growth of OHDSI around the Asia-Pacific region, and plenty more in the latest Collaborator Spotlight.
• The latest edition of the OHDSI newsletter is now available, and it includes the December video presentations of the year in review and OHDSI’s first 10 years. It also features community updates, December publications and presentations, and plenty more.
• Research from the 2023 Global Symposium Collaborator Showcase can be viewed on the Global Symposium Showcase page. Research is also being shared daily on OHDSI’s LinkedIn, Twitter/X and Instagram feeds as part of the #OHDSISocialShowcase. Below are posters (with study leads) that are featured this week:
Monday — A Toxin Vocabulary for the OMOP CDM Park (Maksym Trofymenko)
Tuesday — Developing a perinatal expansion table for the OMOP common data model (Alicia Abellan)
Wednesday — External validation using clinical domain knowledge from the SNOMED medical terms hierarchy (Henrik John)
Thursday — Estimating the comparative risk of kidney failure associated with intravitreal anti-vascular endothelial growth factor (anti-VEGF) exposure in patients with blinding diseases (Cindy Cai)
Friday — Characteristics and outcomes of over a million inflammatory bowel disease subjects in seven countries: a multinational cohort study (Chen Yanover)
HADES Development Updates
• Martiijn Schuemie announced the releases of CohortMethod 5.2.0 and EmpiricalCalibration 3.1.2. The CohortMethod release focuses on generalizability, by comparing the population after all adjustments (e.g. PS matching) to the population before all adjustments, and report any characteristics that have changed. The choice of population to consider for generalizability is now driven by your analysis choices. For example, if you use PS matching, (implying ATT), generalizability will be computed for the target (treated) population.
• Egill Fridgeirsson announced the release of DeepPatientLevelPrediction 2.0.3. This bugfix release only includes one change which was necessary because one of my dependencies introduced a breaking change (polars) which broke some functionality in the package.
• Joel Swerdel announced the release of PheValuator 2.2.11. This adds the capability to include multiple visits per subject in the evaluation cohort.
Job Openings
• There is an opening for a Data Steward position at the EBMT. Among the responsibilities is the design, implementation and testing of new data collection processes including data collection forms (DCFs) development, as well as the mapping of new items from DCFs to the OMOP CDM.
Slides
Main Presentation | Community Updates